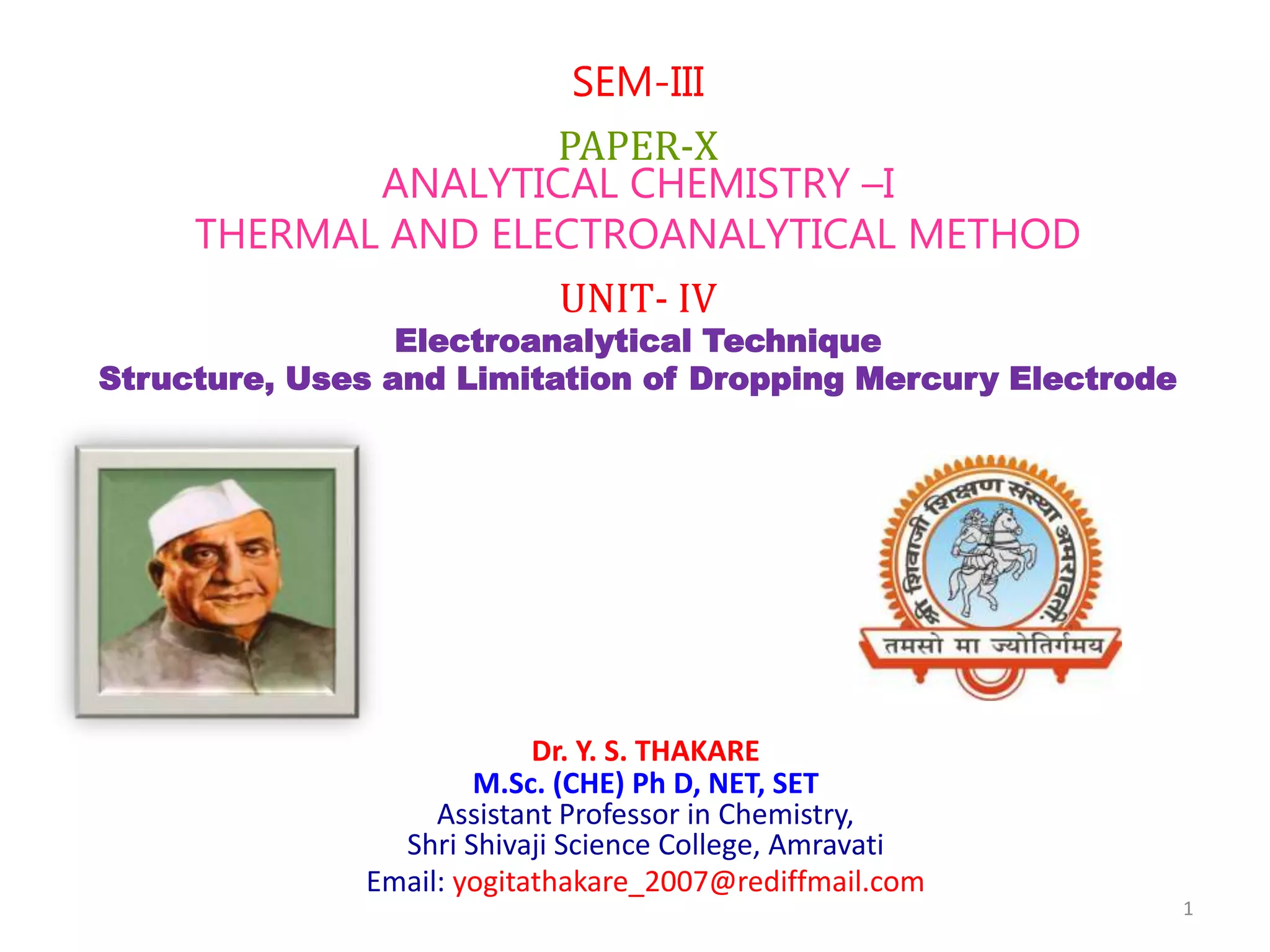
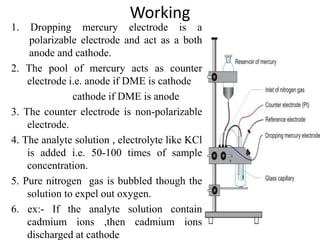
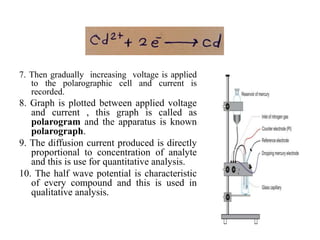
The document discusses the dropping mercury electrode (DME), which is a working electrode used in polarography where mercury drops from a reservoir through a capillary tube into the solution being analyzed. It provides details on the structure of the DME including the mercury reservoir and capillary tube. The document also describes how the DME works, lists different types of mercury electrodes, and discusses the advantages, disadvantages, and precautions of using the DME.



![1] Mercury reservoir
It consist of mercury reservoir bulb
about 100 ml capacity.
The reservoir is clamped in vertical
position.
It connected by flexible tubing to a
glass capillary tube.
The mercury reservoir is raised to
appropriate height on the stand and its
position is marked.
The position / height of the reservoir
should be kept the same throughout
the experiment to get reproducible
results.
Structure of DME](https://image.slidesharecdn.com/lect-210216073144/85/Lect-2-DMG-structure-uses-Advantages-limitation-5-320.jpg)
![2] Capillary tube
The glass tube is ≈7 cm in length and 0.5 mm
in diameter.
One end of the capillary tube is attached to
the flexible tube and clamped. The other end
of the capillary tube dips under the surface of
the solution to be analysed(electrolyte).
The drops of mercury come out from the
capillary continuously.
The drops of mercury are identical with
diameters of ≈0.5 to 1mm.
The life time of an individual drop is 3 to 6
seconds.
The DME is here shown as the cathode
The DME is called as the working electrode
or microelectrode or the indicator
electrode.](https://image.slidesharecdn.com/lect-210216073144/85/Lect-2-DMG-structure-uses-Advantages-limitation-6-320.jpg)
![Types of Mercury electrode
1] DME(dropping mercury electrode)
The mercury drop forms at the end of the
capillary through gravity. It grows
continuously as the mercury flows the
reservoir and has a finite lifetime of several
seconds. It is then dislodged and replaced by
a new drop.
2] SMDE (Static Mercury Drop Electrode)
This electrode uses a solenoid plunger to
control the flow of mercury. Activation of the
solenoid momentarily lifts the plunger ,
allowing mercury to flow through the
capillary forming a single hanging Hg-drop.
3] HDME (Hanging Mercury
drop electrode)
The drop of mercury is extended by rotating a
micrometer screw that pulses the mercury
from a reservoir through a narrow capillary.](https://image.slidesharecdn.com/lect-210216073144/85/Lect-2-DMG-structure-uses-Advantages-limitation-9-320.jpg)


