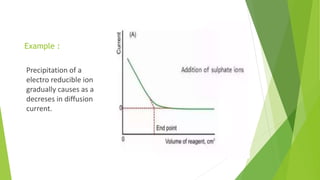
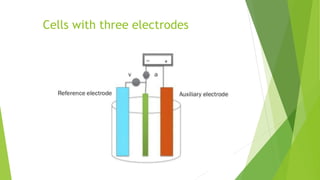
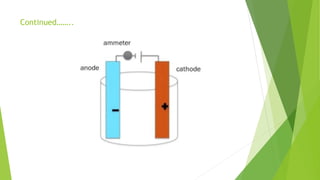
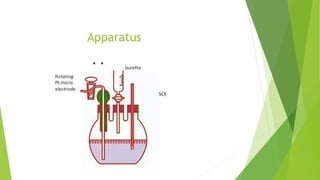
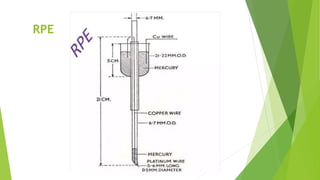
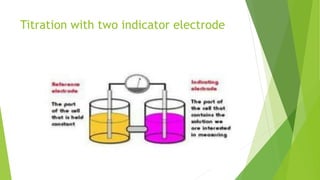
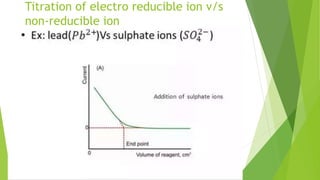
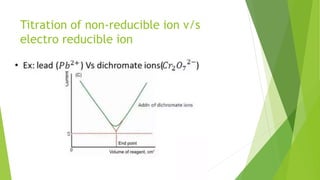
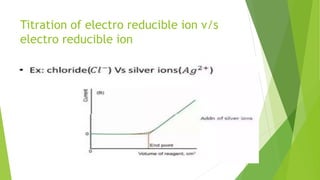
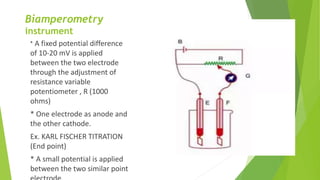
Amperometric titration involves measuring the electric current produced by a titration reaction while keeping the voltage constant between electrodes. It can determine the endpoint of titrations involving an electroreducible ion being titrated with a counter ion. The diffusion current is measured and plotted against the titrant volume added. At the endpoint, there is a sharp change in current. Amperometric titration offers advantages like rapid analysis, ability to work with dilute solutions, and determination of insoluble substances. It finds applications in areas like determining water content and quantification of ions.