Embed presentation
Downloaded 65 times
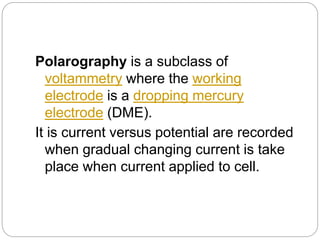
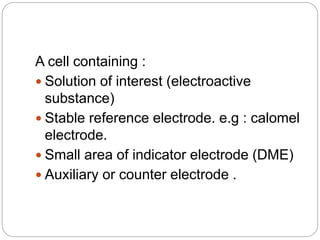
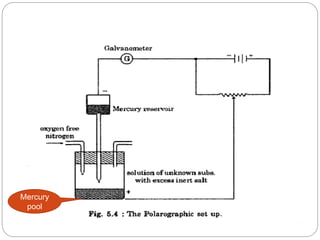



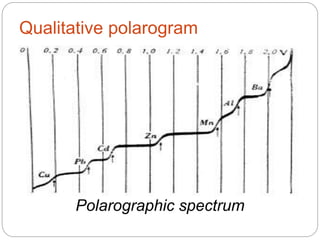
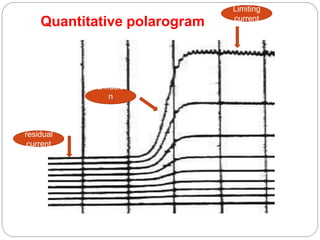
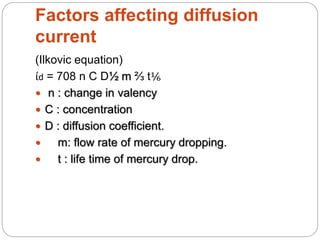
Polarography is a type of voltammetry where a dropping mercury electrode is used as the working electrode. A polarography cell contains an electrolyte solution, a reference electrode like calomel, a dropping mercury electrode, and an auxiliary electrode. As a potential is applied, the current is measured as mercury droplets fall through the solution. This allows for the analysis of electroactive substances and the production of polarograms that can be used for qualitative and quantitative analysis based on diffusion currents.









