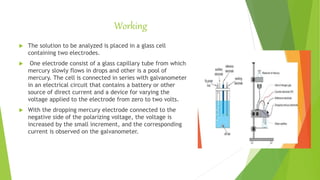
Polarography is an electrochemical technique used to analyze reducible or oxidizable substances in solution. It involves varying the electric potential between a dropping mercury electrode and a reference electrode while monitoring the current. A polarogram is generated by plotting the current readings against the applied voltage. Key features of polarography include applied voltages between 0-2.5V and current values between 0.12-100 microamperes. Polarography finds applications in pharmaceutical analysis such as determining dissolved oxygen, trace metals in drugs, vitamins, hormones, antibiotics, and diagnosing cancer from blood serum.