Embed presentation
Downloaded 45 times





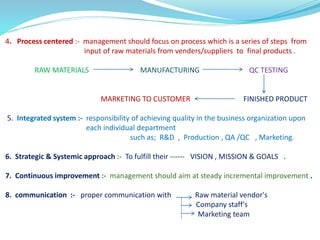
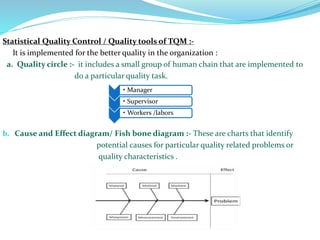
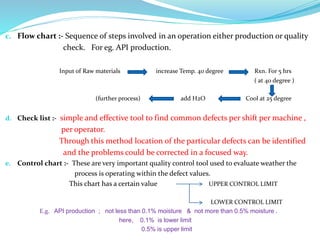





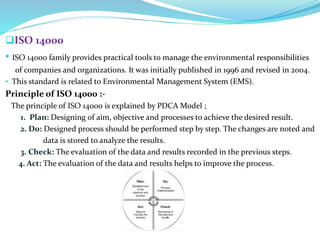



The document discusses quality management systems in the pharmaceutical industry. It provides an overview of key aspects of quality management including Quality by Design (QbD), Total Quality Management (TQM), ISO 9000 and ISO 14000 standards. The main points are: 1) Quality management systems (QMS) rely on regulations and guidelines to ensure product and process quality in the pharmaceutical industry. 2) QbD and TQM approaches aim to increase manufacturing efficiency and product quality through systematic process understanding and employee involvement. 3) International standards like ISO 9000 specify quality management principles for meeting customer and regulatory requirements, while ISO 14000 provides an environmental management system framework.
















