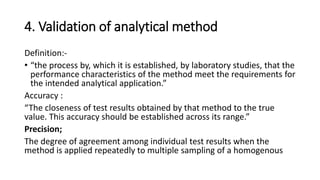
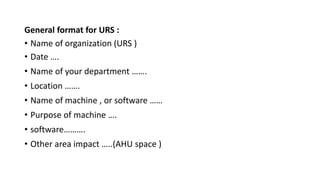
The document discusses different types of validation processes that are important for pharmaceutical manufacturing. It describes process validation, cleaning validation, equipment validation, and validation of analytical methods. Process validation ensures a process is capable of consistently producing quality products and includes prospective, concurrent, retrospective, and revalidation. Cleaning validation aims to minimize cross-contamination. Equipment validation proves equipment works correctly. Validation of analytical methods establishes that test method performance meets requirements for intended use. Government regulations require validation to ensure drug quality and safety.