chapter-6-Science 8-1223664031830946-8.pdf
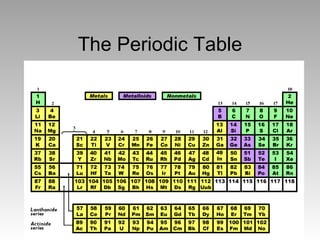
- 2. • Introduction – The periodic table is made up of rows of elements and columns. – An element is identified by its chemical symbol. – The number above the symbol is the atomic number – The number below the symbol is the rounded atomic weight of the element. – A row is called a period – A column is called a group
- 3. Organizing the Elements • Chemists used the properties of elements to sort them into groups. • JW. Dobreiner grouped elements into triads. • A triad is a set of three elements with similar properties.
- 4. Mendeleev’s Periodic Table • In 1869, a Russian chemist and teacher published a table of the elements. • Mendeleev arranged the elements in the periodic table in order of increasing atomic mass.
- 5. Henry Moseley 1887 - 1915 In 1913, through his work with X-rays, he determined the actual nuclear charge (atomic number) of the elements*. He rearranged the elements in order of increasing atomic number. *“There is in the atom a fundamental quantity which increases by regular steps as we pass from each element to the next. This quantity can only be the charge on the central positive nucleus.”
- 6. The Periodic Law In the modern periodic table elements are arranged in order of increasing atom ic num ber. Periodic Law states: When elements are arranged in order of increasing atomic number, there is a periodic repetition of their physical and chemical properties.
- 7. • The elements can be grouped into three broad classes based on their general properties. • Three classes of elements are Metals, Nonmetals, and Metalloids. • Across a period, the properties of elements become less metallic and more nonmetallic.
- 8. Properties of Metals • Metals are good conductors of heat and electricity. • Metals are shiny. • Metals are ductile (can be stretched into thin wires). • Metals are malleable (can be pounded into thin sheets). • A chemical property of metal is its reaction with water which results in corrosion. • Solid at room temperature except Hg.
- 9. Properties of Non-Metals • Non-metals are poor conductors of heat and electricity. • Non-metals are not ductile or malleable. • Solid non-metals are brittle and break easily. • They are dull. • Many non-metals are gases. Sulfur
- 10. Properties of Metalloids • Metalloids (metal-like) have properties of both metals and non-metals. • They are solids that can be shiny or dull. • They conduct heat and electricity better than non- metals but not as well as metals. • They are ductile and malleable. Silicon
- 11. Groups Periods Groups Periods Columns of elements are Columns of elements are called groups or families. called groups or families. Elements in each group Elements in each group have similar but not have similar but not identical properties. identical properties. For example, lithium (Li), For example, lithium (Li), sodium (Na), potassium sodium (Na), potassium (K), and other members of (K), and other members of group IA are all soft, white, group IA are all soft, white, shiny metals. shiny metals. All elements in a group All elements in a group have the same number of have the same number of valence electrons. valence electrons. Each horizontal row of Each horizontal row of elements is called a period. elements is called a period. The elements in a period The elements in a period are not alike in properties. are not alike in properties. In fact, the properties In fact, the properties change greatly across even change greatly across even given row. given row. The first element in a period The first element in a period is always an extremely is always an extremely active solid. The last active solid. The last element in a period, is element in a period, is always an inactive gas. always an inactive gas.
- 12. Hydrogen Hydrogen The hydrogen square sits atop group AI, but The hydrogen square sits atop group AI, but it is not a member of that group. Hydrogen is it is not a member of that group. Hydrogen is in a class of its own. in a class of its own. It’s a gas at room temperature. It’s a gas at room temperature. It has one proton and one electron in its one It has one proton and one electron in its one and only energy level. and only energy level. Hydrogen only needs 2 electrons to fill up its Hydrogen only needs 2 electrons to fill up its valence shell. valence shell.
- 13. 6.2 Classifying the Elements 6.2 Classifying the Elements The periodic table The periodic table displays the symbols displays the symbols and names of the and names of the elements along with elements along with information about the information about the structure of their structure of their atoms. atoms.
- 14. Four chemical groups Four chemical groups of the periodic table: of the periodic table: 2. 2. alkali metals (IA) alkali metals (IA) 3. 3. alkaline earth metals alkaline earth metals (IIA), (IIA), 4. 4. Halogens (VII), Halogens (VII), 5. 5. Noble Noble gases gases (VIIIA). (VIIIA).
- 15. Alkali Metals Alkali Metals The alkali family is found in The alkali family is found in the first column of the the first column of the periodic table. periodic table. Atoms of the alkali metals Atoms of the alkali metals have a single electron in their have a single electron in their outermost level, in other outermost level, in other words, 1 valence electron. words, 1 valence electron. They are shiny, have the They are shiny, have the consistency of clay, and are consistency of clay, and are easily cut with a knife. easily cut with a knife.
- 16. Alkali Metals Alkali Metals They are the most They are the most reactive metals. reactive metals. They react violently They react violently with water. with water. Alkali metals are Alkali metals are never found as free never found as free elements in nature. elements in nature. They are always They are always bonded with another bonded with another element. element.
- 17. Alkaline Earth Metals Alkaline Earth Metals They are never found uncombined in nature. They are never found uncombined in nature. They have two valence electrons. They have two valence electrons. Alkaline earth metals include magnesium and Alkaline earth metals include magnesium and calcium, among others. calcium, among others.
- 18. Transition Metals Transition Metals Transition Elements Transition Elements include those elements in include those elements in the B groups. the B groups. These are the metals you These are the metals you are probably most are probably most familiar: copper, tin, zinc, familiar: copper, tin, zinc, iron, nickel, gold, and iron, nickel, gold, and silver. silver. They are good conductors They are good conductors of heat and electricity. of heat and electricity.
- 19. Transition Metals Transition Metals The compounds of transition metals are usually brightly The compounds of transition metals are usually brightly colored and are often used to color paints. colored and are often used to color paints. Transition elements have 1 or 2 valence electrons, which Transition elements have 1 or 2 valence electrons, which they lose when they form bonds with other atoms. Some they lose when they form bonds with other atoms. Some transition elements can lose electrons in their next-to- transition elements can lose electrons in their next-to- outermost level. outermost level.
- 20. Transition Elements Transition Elements Transition elements Transition elements have properties have properties similar to one another and to other metals, similar to one another and to other metals, but their properties do not fit in with those but their properties do not fit in with those of any other group. of any other group. Many transition metals combine Many transition metals combine chemically with oxygen to form chemically with oxygen to form compounds called oxides. compounds called oxides.
- 21. Representative Elements Representative Elements Groups 1A – 7A. Groups 1A – 7A. Elements are refered to as representative Elements are refered to as representative elements because they display a wide elements because they display a wide range of physical and chemical properties. range of physical and chemical properties. For any representative element, its group For any representative element, its group number equals the number of electrons in number equals the number of electrons in the highest occupied energy level. the highest occupied energy level.
- 22. Trends in the periodic Trends in the periodic table: table: Ionization Energy Ionization Energy Atomic Radius Atomic Radius Electron Affinity Electron Affinity Electronegativity Electronegativity
- 23. Sizes of Atoms Sizes of Atoms The bonding atomic The bonding atomic radius is defined as radius is defined as one-half of the one-half of the distance between distance between covalently bonded covalently bonded nuclei. nuclei.
- 24. Atomic Radius Trend Atomic Radius Trend Group Trend – As you go Group Trend – As you go down a column down a column, , atomic radius increases. atomic radius increases. As you go down, e As you go down, e- - are filled into orbitals that are are filled into orbitals that are farther away from the nucleus (attraction not farther away from the nucleus (attraction not as strong). as strong). Periodic Trend – As you go Periodic Trend – As you go across a period across a period (L (L to R), to R), atomic radius decreases. atomic radius decreases. As you go L to R, e As you go L to R, e- - are put into the same orbital, are put into the same orbital, but more p but more p+ + and e and e- - total (more attraction = total (more attraction = smaller size). smaller size).
- 27. Ionic Radius Trend Ionic Radius Trend Metals Metals – lose e – lose e- - , which means more p , which means more p+ + than e than e- - (more attraction) SO… (more attraction) SO… Ionic Radius Ionic Radius < < Neutral Atomic Radius Neutral Atomic Radius Nonmetals Nonmetals – gain e – gain e- - , which means more e , which means more e- - than p than p+ + (not as much attraction) SO… (not as much attraction) SO… Ionic Radius Ionic Radius > > Neutral Atomic Radius Neutral Atomic Radius
- 28. Sizes of Ions Sizes of Ions Ionic size depends Ionic size depends upon: upon: Nuclear charge. Nuclear charge. Number of Number of electrons. electrons. Orbitals in which Orbitals in which electrons reside. electrons reside.
- 29. Sizes of Ions Sizes of Ions Cations are Cations are smaller than their smaller than their parent atoms. parent atoms. The outermost The outermost electron is electron is removed and removed and repulsions are repulsions are reduced. reduced.
- 30. Sizes of Ions Sizes of Ions Anions are larger Anions are larger than their parent than their parent atoms. atoms. Electrons are Electrons are added and added and repulsions are repulsions are increased. increased.
- 31. Sizes of Ions Sizes of Ions Ions increase in size Ions increase in size as you go down a as you go down a column. column. Due to increasing Due to increasing value of value of n n. .
- 32. Metals versus Nonmetals Metals versus Nonmetals Metals tend to form cations. Metals tend to form cations. Nonmetals tend to form anions. Nonmetals tend to form anions.
- 33. Background Background Electrons can jump between shells (Bohr’s Electrons can jump between shells (Bohr’s model supported by line spectra) model supported by line spectra) The electrons can be pushed so far that The electrons can be pushed so far that they escape the attraction of the nucleus they escape the attraction of the nucleus Losing an electron is called ionization Losing an electron is called ionization An ion is an atom that has either a net An ion is an atom that has either a net positive or net negative charge positive or net negative charge Q: what would the charge be on an atom Q: what would the charge be on an atom that lost an electron? Gained two electrons? that lost an electron? Gained two electrons? A: +1 (because your A: +1 (because your losing losing a -ve electron) a -ve electron) A: -2 (because you gain 2 -ve electrons) A: -2 (because you gain 2 -ve electrons)
- 34. Ionization Energy Ionization Energy Amount of energy required to remove an Amount of energy required to remove an electron from the ground state of a electron from the ground state of a gaseous atom or ion. gaseous atom or ion. First ionization energy is that energy required First ionization energy is that energy required to remove first electron. to remove first electron. Second ionization energy is that energy Second ionization energy is that energy required to remove second electron, etc. required to remove second electron, etc.
- 35. Ionization Energy Ionization Energy Group Trend – As you go Group Trend – As you go down a column down a column, , ionization energy decreases. ionization energy decreases. As you go down, atomic size is increasing (less As you go down, atomic size is increasing (less attraction), so easier to remove an e attraction), so easier to remove an e- - . . Periodic Trend – As you go Periodic Trend – As you go across a period across a period (L to (L to R), R), ionization energy increases. ionization energy increases. As you go L to R, atomic size is decreasing (more As you go L to R, atomic size is decreasing (more attraction), so more difficult to remove an e attraction), so more difficult to remove an e- - (also, metals want to lose e (also, metals want to lose e- - , but nonmetals do , but nonmetals do not). not).
- 36. Ionization Energy Ionization Energy It requires more energy to remove each It requires more energy to remove each successive electron. successive electron. When all valence electrons have been removed, When all valence electrons have been removed, the ionization energy takes a quantum leap. the ionization energy takes a quantum leap.
- 37. Trends in First Ionization Trends in First Ionization Energies Energies As one goes down a As one goes down a column, less energy column, less energy is required to remove is required to remove the first electron. the first electron. For atoms in the same For atoms in the same group, group, Z Zeff eff is essentially is essentially the same, but the the same, but the valence electrons are valence electrons are farther from the farther from the nucleus. nucleus.
- 38. Electronegativity Electronegativity Electronegativity- Electronegativity- tendency of an tendency of an atom to attract e atom to attract e- - . .
- 39. Electronegativity Trend Electronegativity Trend Group Trend – As you go Group Trend – As you go down a column down a column, , electronegativity decreases. electronegativity decreases. As you go down, atomic size is increasing, so less As you go down, atomic size is increasing, so less attraction to its own e attraction to its own e- - and other atom’s e and other atom’s e- - . . Periodic Trend – As you go Periodic Trend – As you go across a period across a period (L to R), (L to R), electronegativity increases. electronegativity increases. As you go L to R, atomic size is decreasing, so there is As you go L to R, atomic size is decreasing, so there is more attraction to its own e more attraction to its own e- - and other atom’s e and other atom’s e- - . .