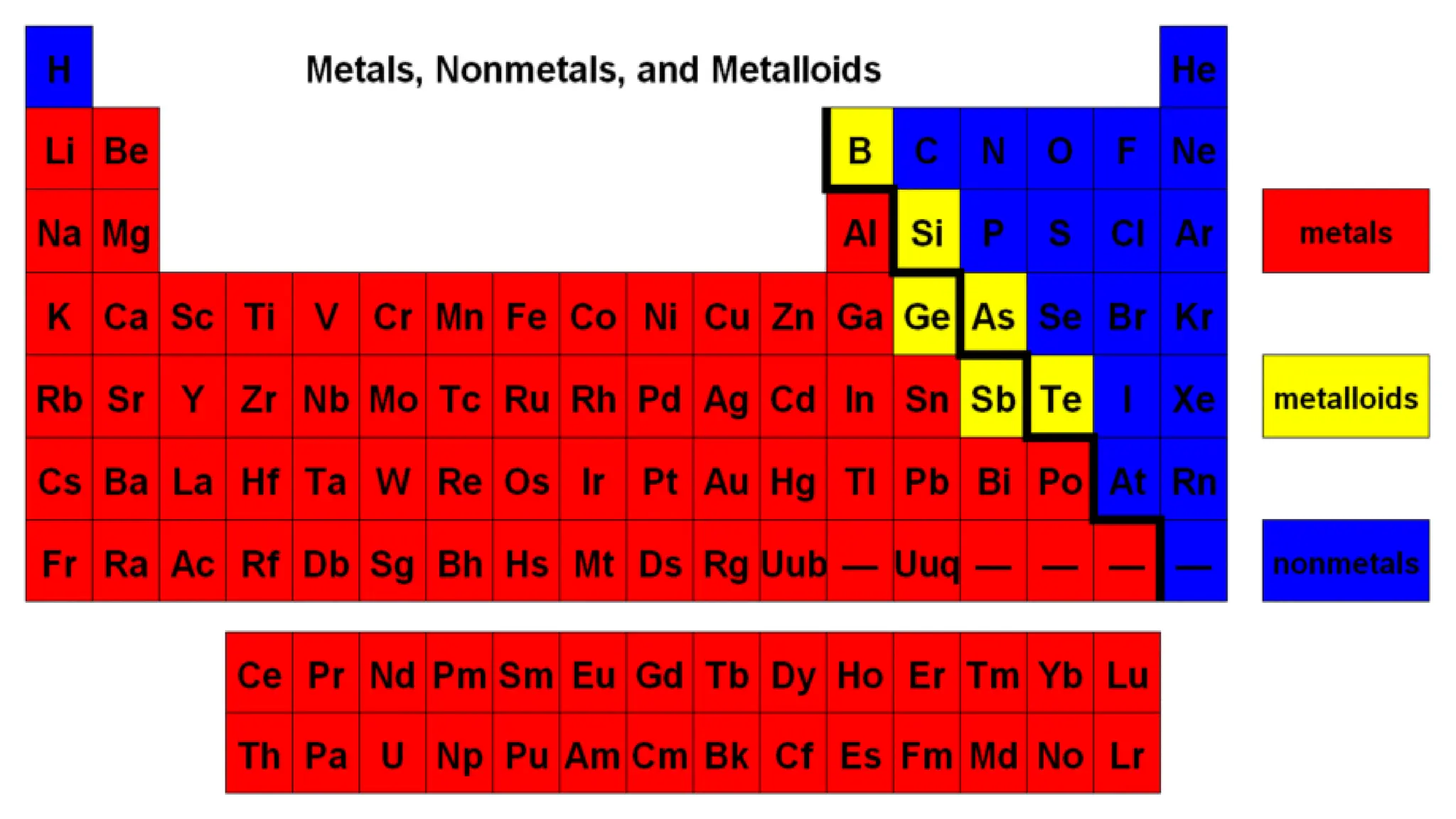
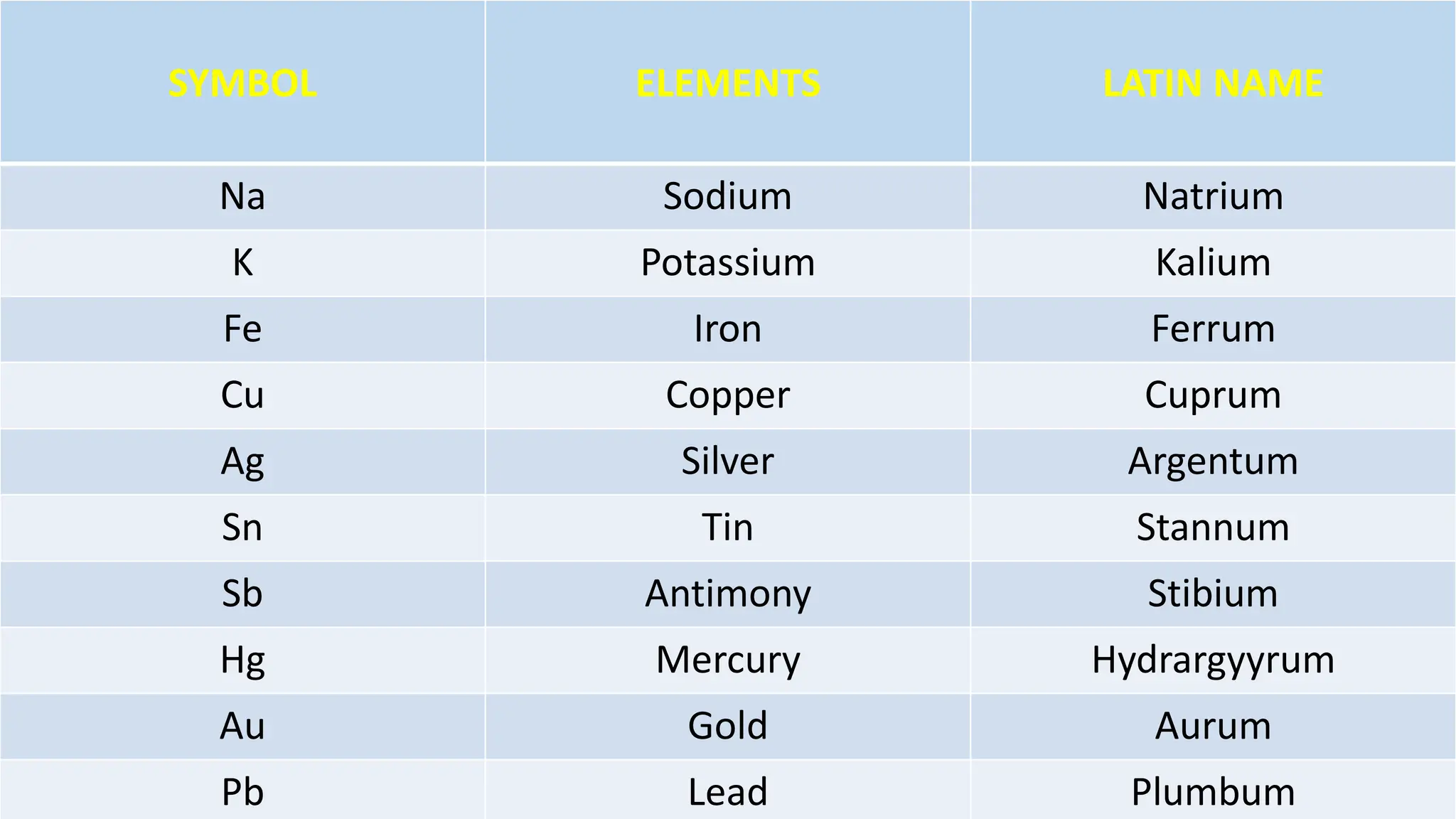
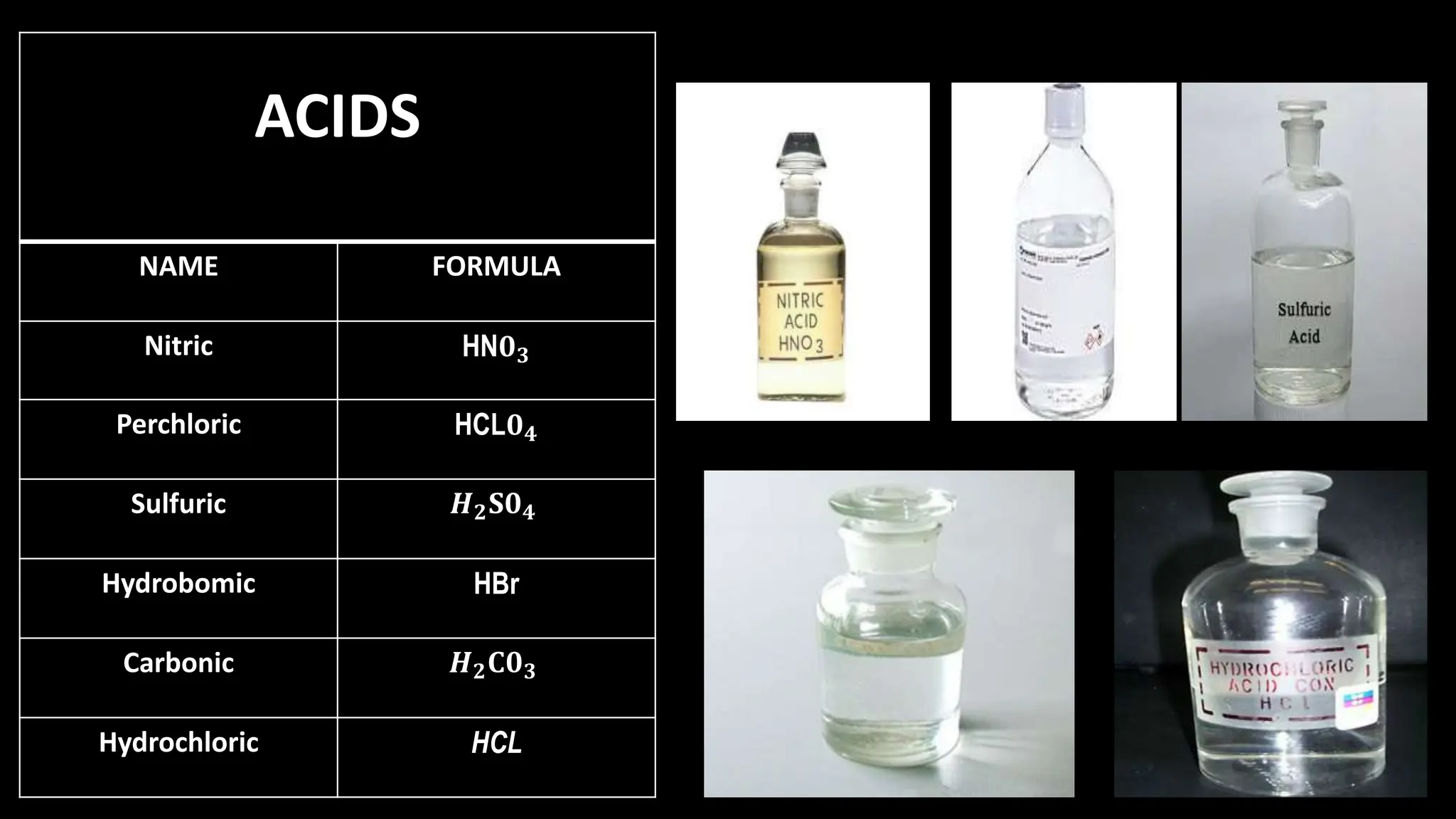
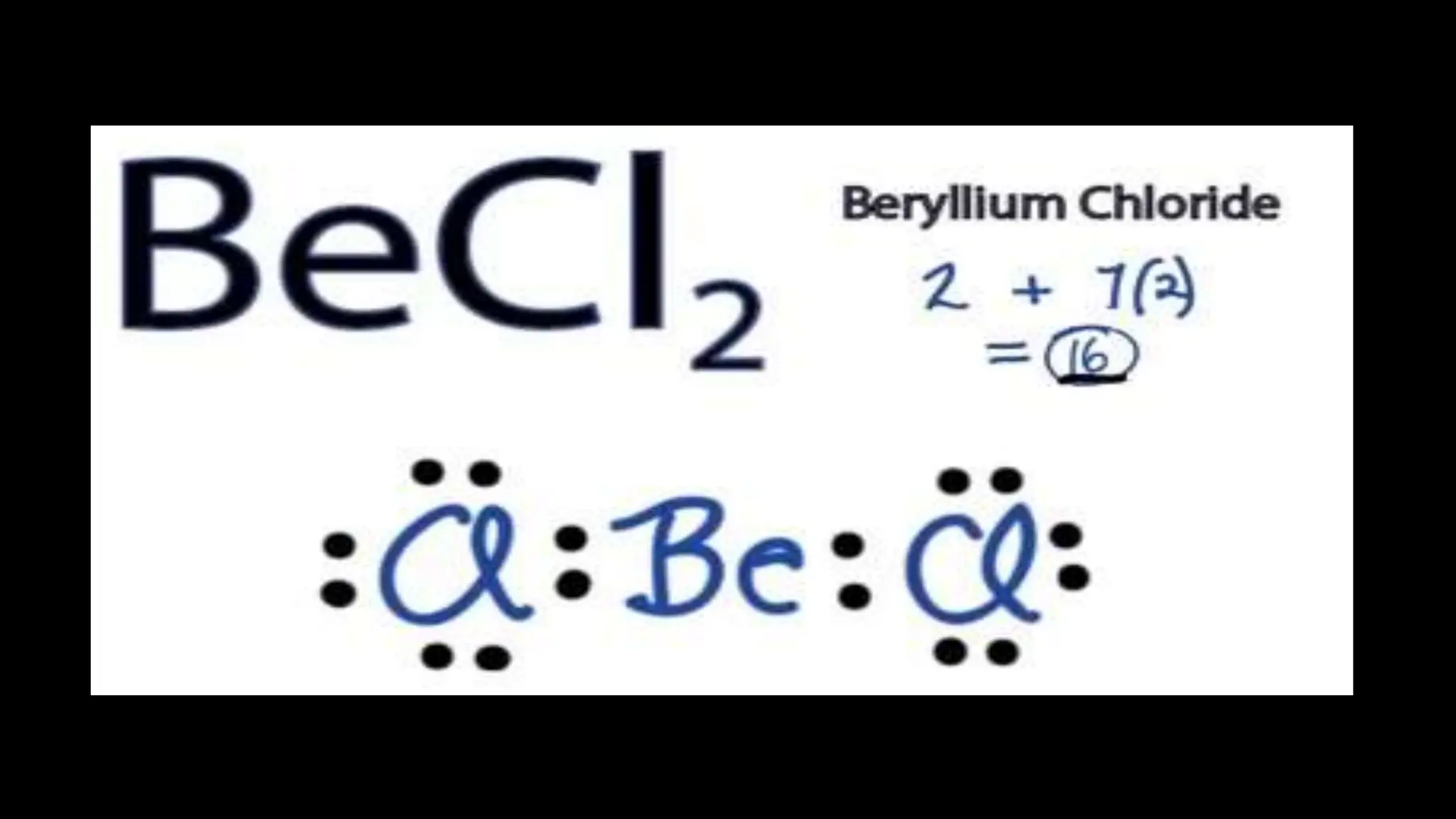
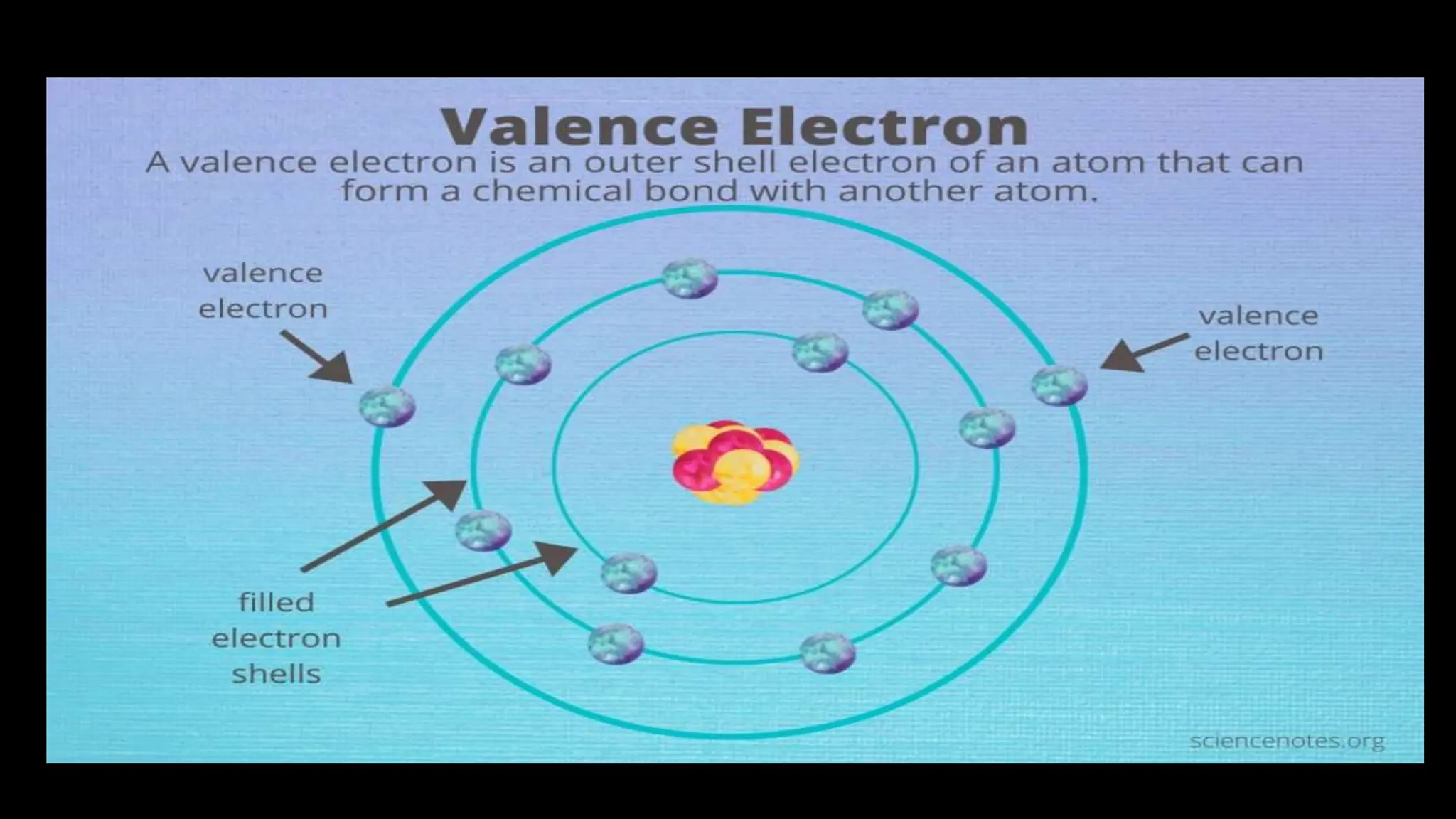
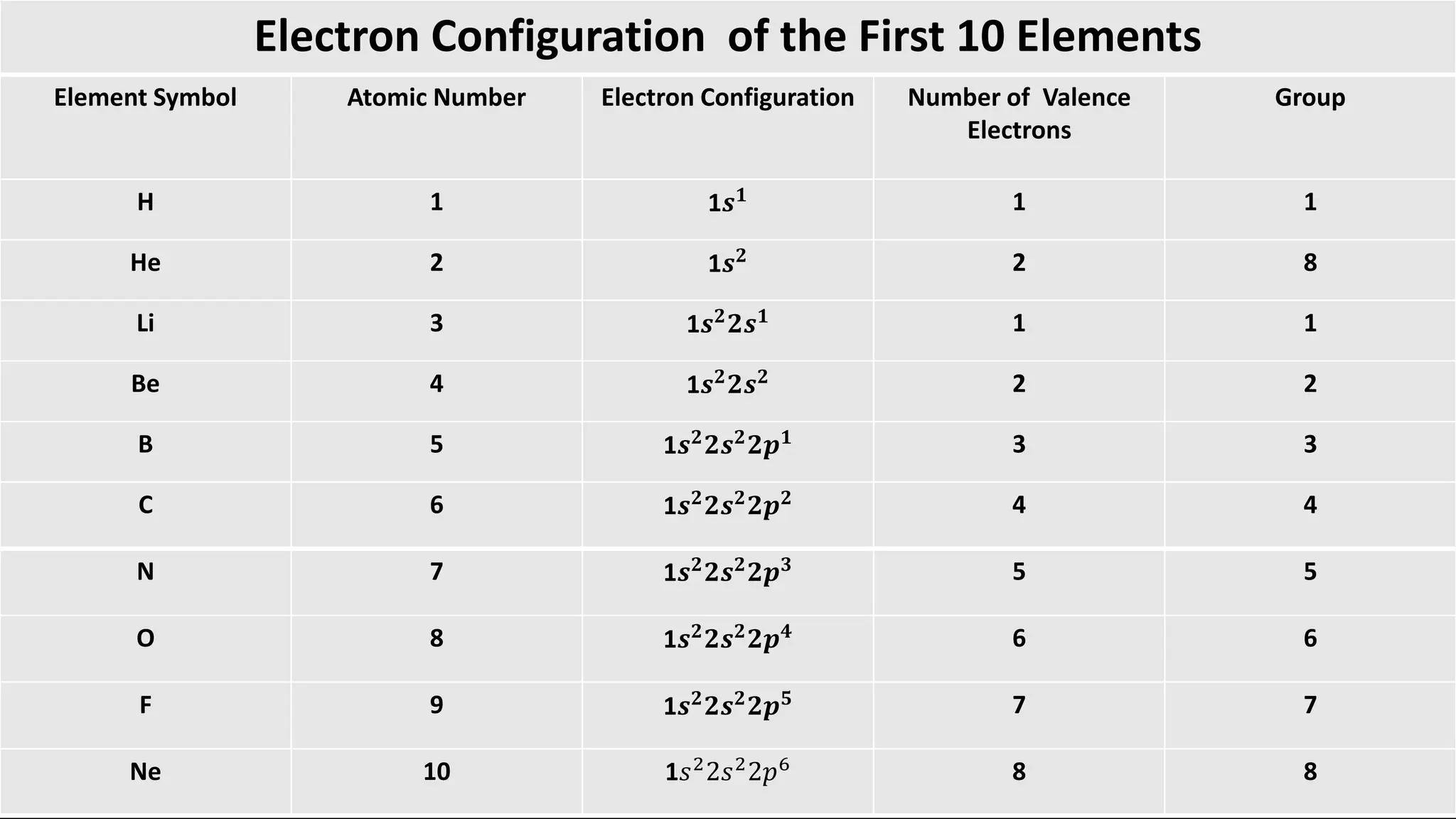
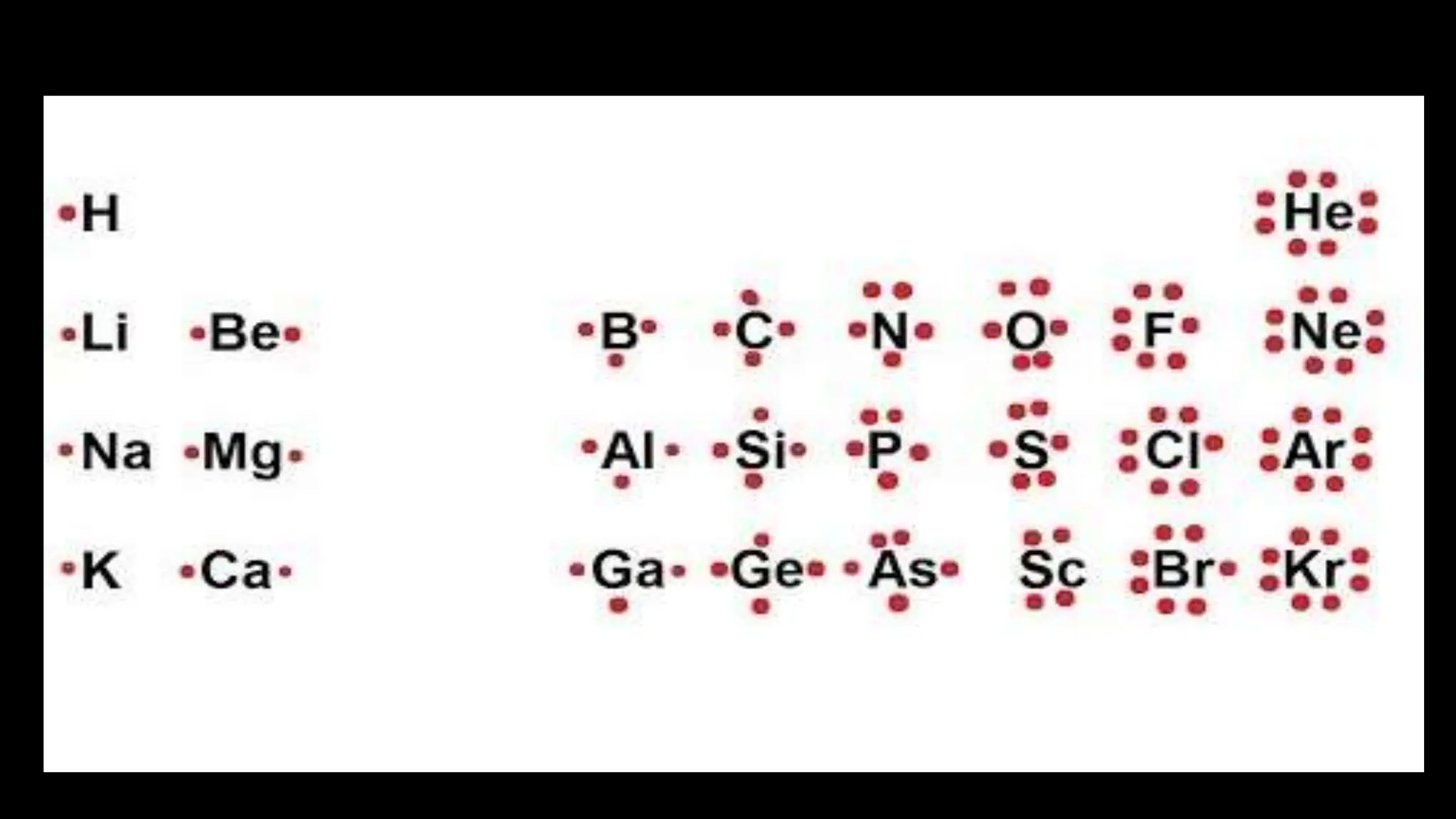
Chemical bonding occurs through either ionic bonds or covalent bonds. Ionic bonds result from the electrostatic attraction between oppositely charged ions, while covalent bonds form through the sharing of electrons between atoms. The octet rule states that atoms seek to attain a full outer shell of eight electrons to achieve stability. Covalent bonds form when atoms share electrons to attain stable electron configurations of eight electrons. Metallic bonding is characterized by positive metal ions embedded in a "sea" of delocalized valence electrons, giving rise to metallic properties such as conductivity, malleability, and luster.