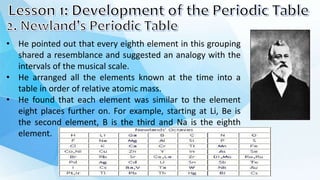
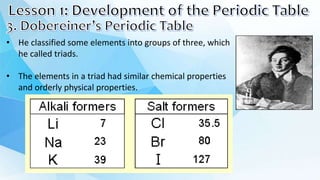
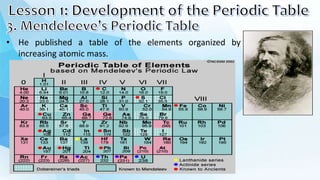
1. Dmitri Mendeleev created one of the first periodic tables by arranging the elements in order of increasing atomic mass. He noticed that elements seemed to repeat properties every eighth element.
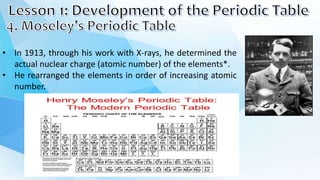
2. Mendeleev arranged the elements into groups with similar properties and left gaps for elements that had not yet been discovered. The periodic table was later reorganized by atomic number.
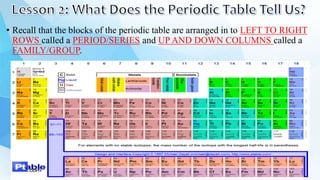
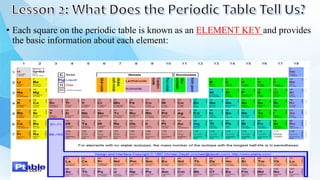
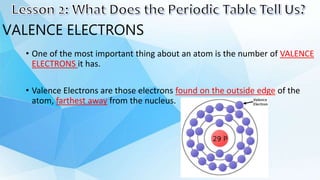
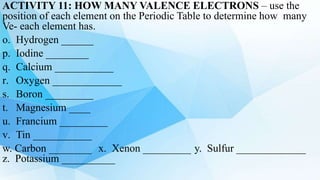
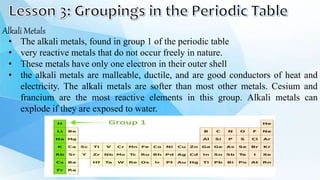
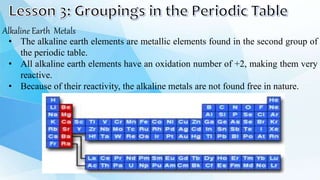
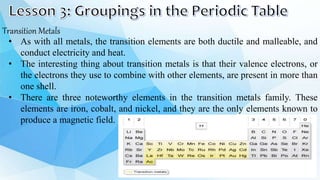
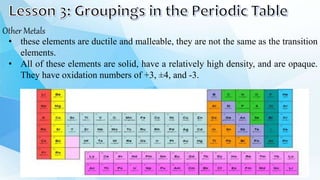
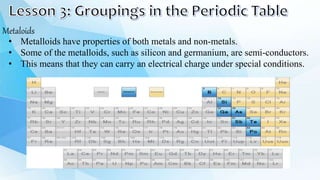
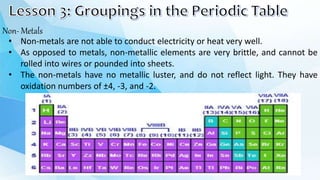
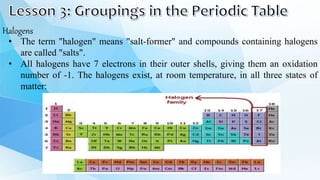
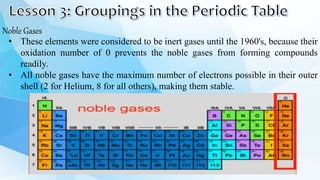
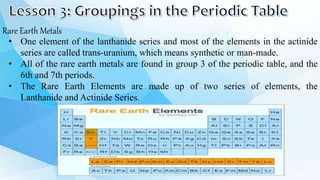
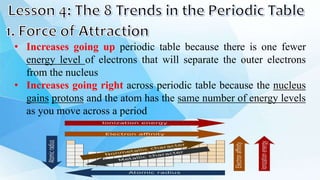
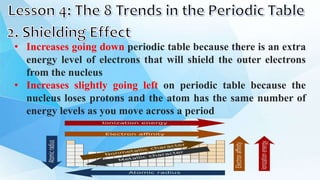
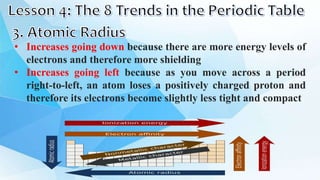
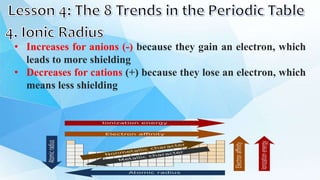
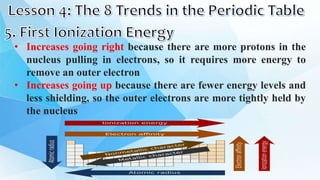
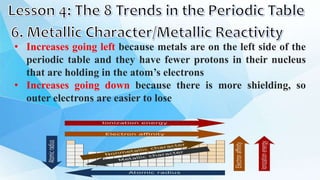
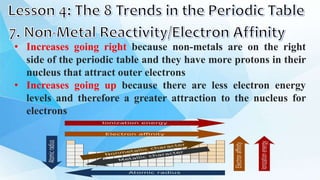
3. The periodic table is arranged into rows called periods and columns called groups. Each element square provides the symbol, name, atomic number and other properties. Valence electrons are important for determining chemical properties and are indicated by the group number.