Embed presentation
Download as PDF, PPTX

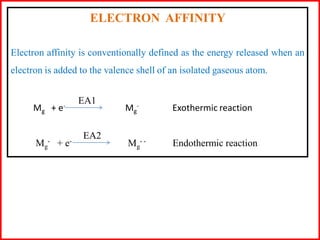
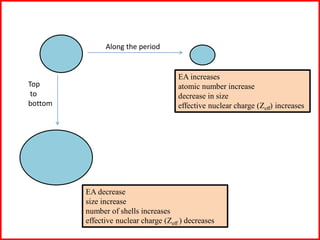
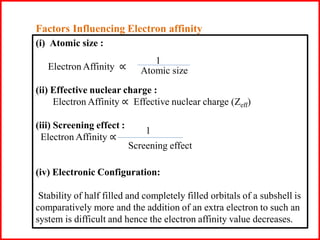
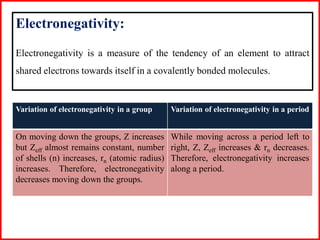
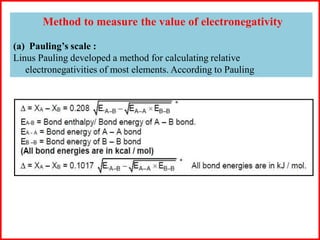
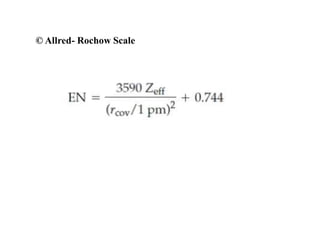
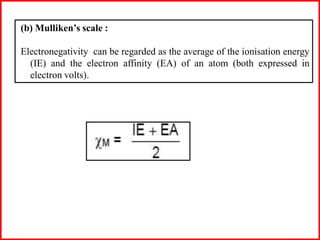

Electron affinity is the energy released when an electron is added to an isolated gaseous atom. Electron affinity increases with increasing atomic number and decreasing size, as effective nuclear charge increases. Electron affinity decreases with increasing size and number of electron shells, as effective nuclear charge decreases. Electron affinity also increases with greater effective nuclear charge and decreases with greater screening effects and stability of half or completely filled orbitals.








