Carboxylic acids contain a carboxyl group, which consists of a carbonyl group bonded to a hydroxyl group. Aliphatic acids have an alkyl group bonded to the carboxyl carbon, while aromatic acids have an aryl group. Common examples are acetic acid (CH3COOH) and benzoic acid (C6H5COOH). Carboxylic acids are named using the IUPAC system, which numbers the carboxyl carbon as number 1 and includes prefixes to identify branches. They can be prepared through oxidation of primary alcohols and aromatics, hydrolysis of nitriles and esters, carbonation of Grignard reagents, or ozonolysis of alkynes.


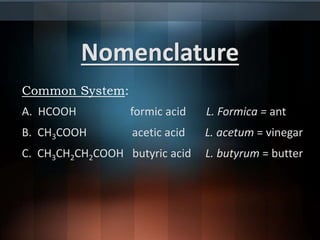

![Nomenclature (cont.)
F. Functional groups priority : -COOH > -COOR
> -COX > -CONH2 > -CN > -CHO > -CO- > -OH
> - NH2 > -OR > -C=C- > -X > -NO2
CH3- CH(CH3)-CH(OH)-COOH
[ 2-hydroxy-3-methyl-butanoic acid ]
CH3-CH=CH-CH2-COOH
[ Pent-3-en-1-oic Acid ]](https://image.slidesharecdn.com/carboxylicacid-170722045933/85/Carboxylic-Acid-5-320.jpg)

