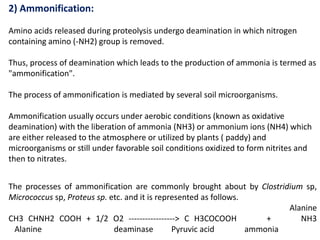
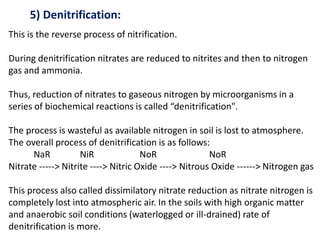
The document discusses the significance of nitrogen cycling in soil, detailing how living organisms depend on fixed nitrogen, primarily from microorganisms. It elaborates on various processes involved in nitrogen fixation, including proteolysis, ammonification, nitrification, and denitrification, emphasizing the roles of specific bacteria in these processes. It concludes that while denitrification reduces soil nitrogen, it serves an ecological purpose by preventing toxic nitrate accumulation.