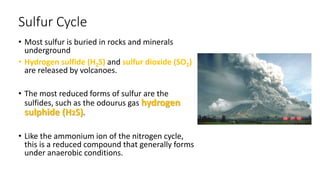
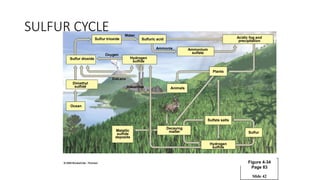
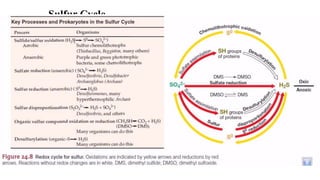
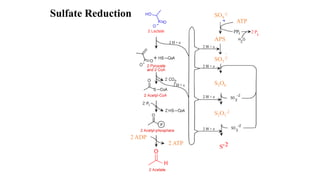
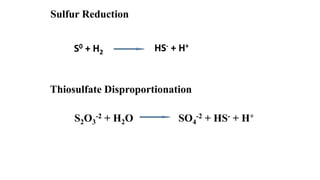
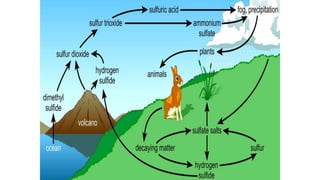
The sulfur cycle describes the movement of sulfur through the biosphere and lithosphere. Key points include:
- Sulfur is released by volcanoes as hydrogen sulfide and sulfur dioxide gases.
- Sulfur is oxidized to sulfate and reduced to sulfide as it moves between organisms and the environment.
- Human activities like burning fossil fuels release sulfur dioxide into the atmosphere.