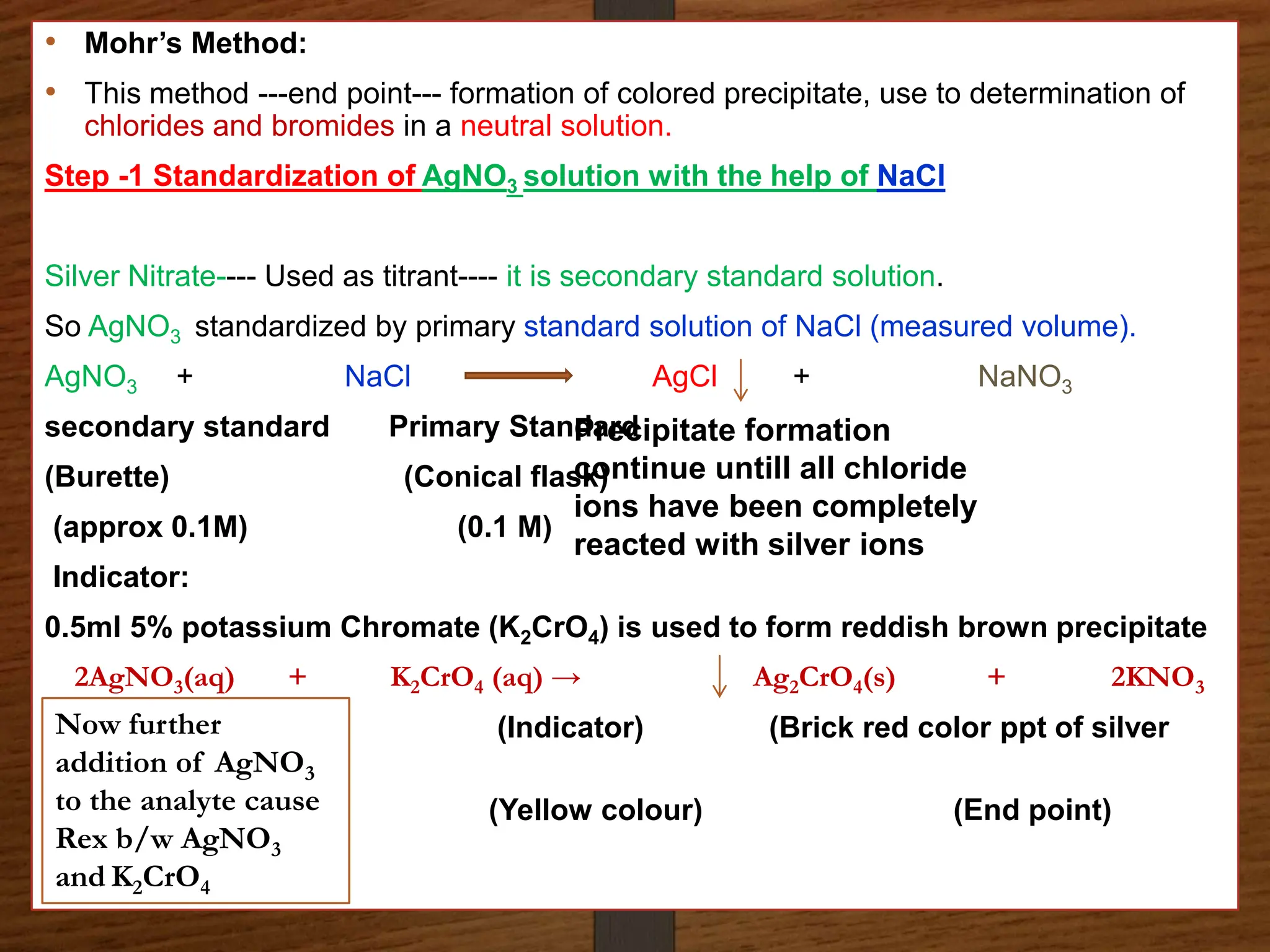
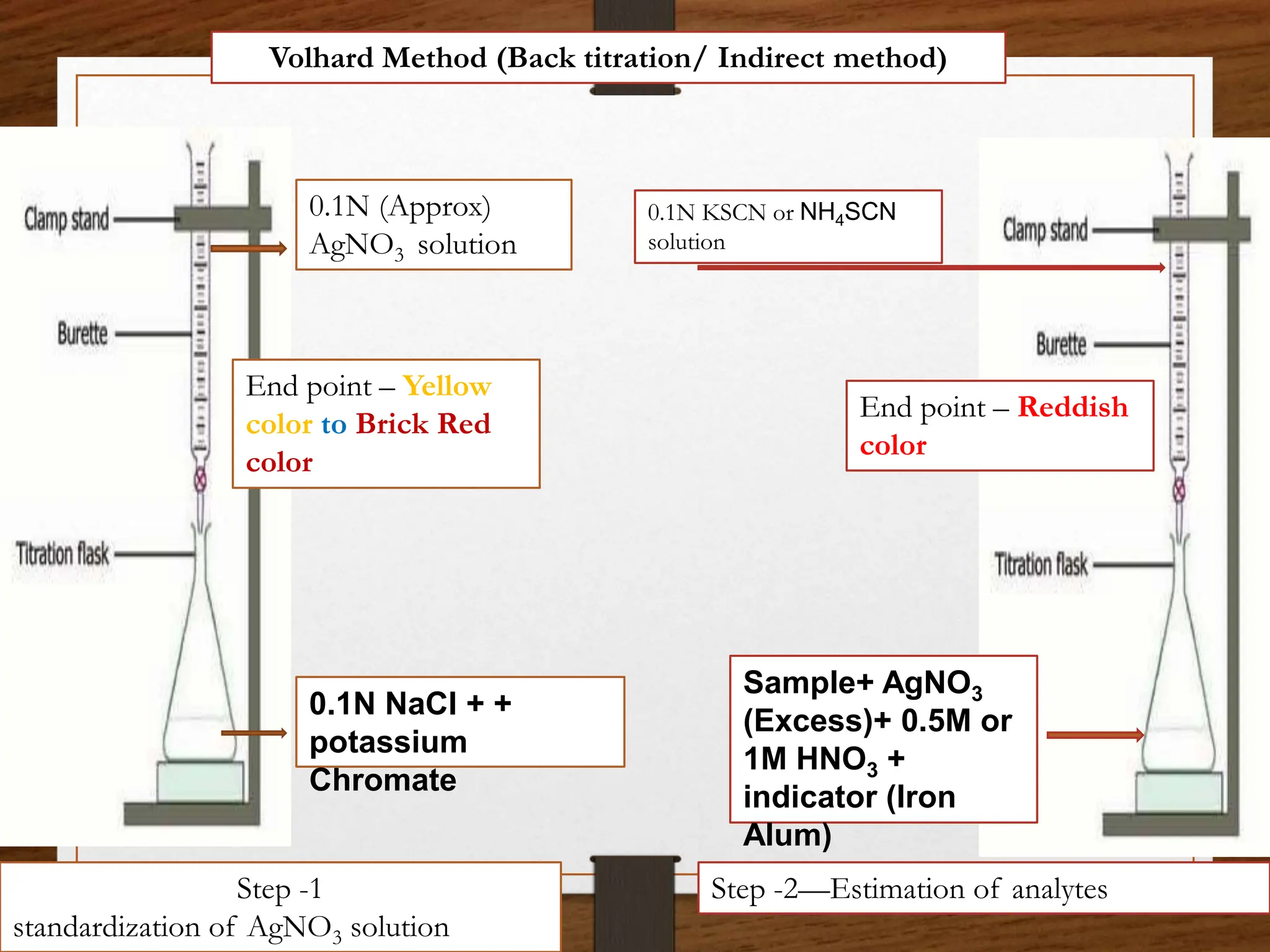
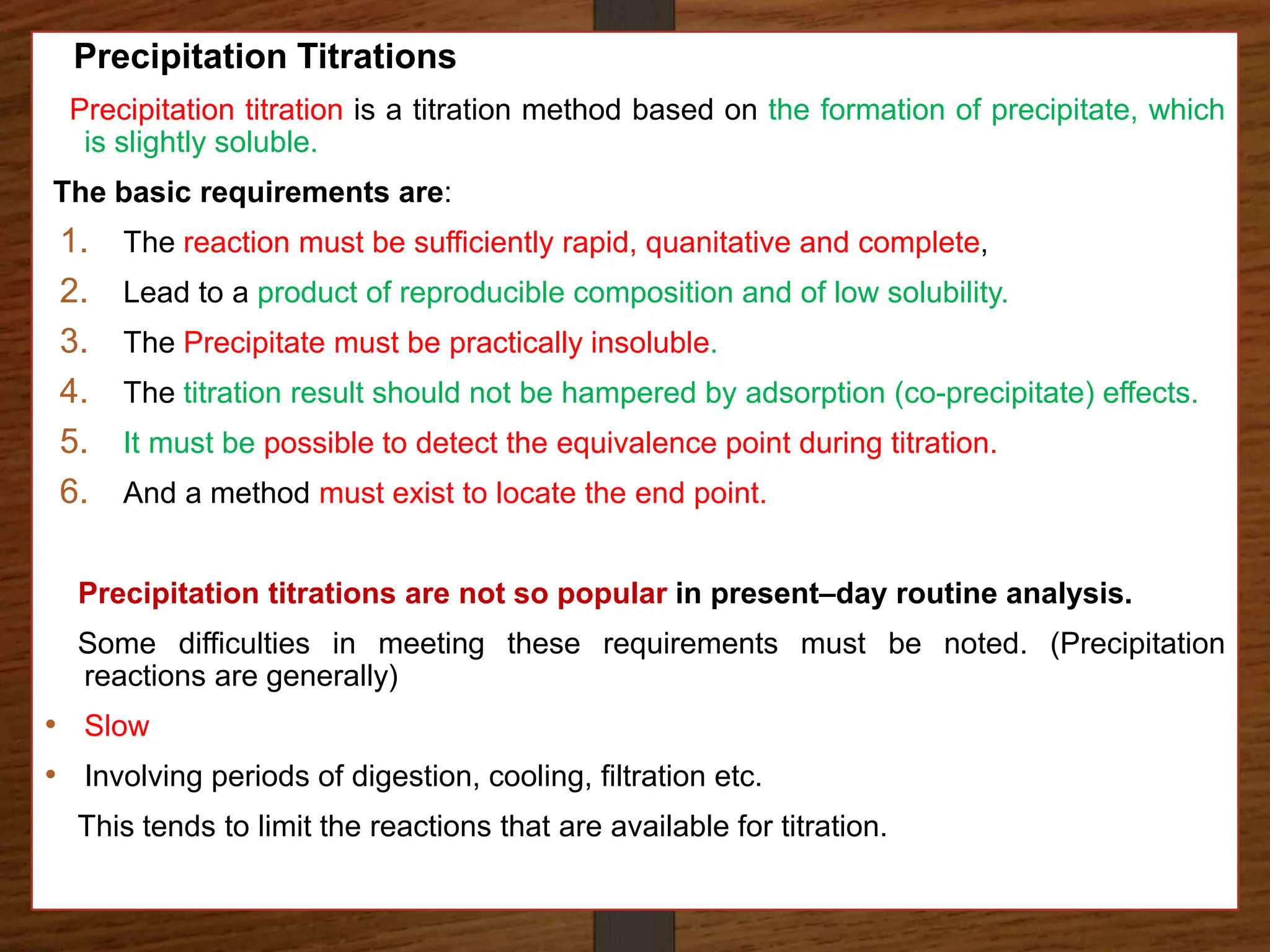
The document discusses various methods of precipitation titration in pharmaceutical chemistry, primarily focusing on argentometric titrations, including Mohr's, Volhard's, and Fajans methods. It outlines the principles of each method, requirements for successful titration, and specific procedures for determining chloride ions' concentration in solutions. Additionally, it highlights limitations and optimal conditions for conducting these titrations, as well as the importance of indicators and maintaining appropriate pH levels.




![ The chloride ion concentration of the MgCl2 and CaCl2 solutions is
determined by a precipitation titration with calibrated silver nitrate
solution. This procedure is known as Mohr's method.
Note- AR Silver nitrate has purity of at least 99.9% so that standard solution
can be prepared by direct weighing
AR Sodium chloride- has purity of 99.9 to 100%; therefore an substance is
therefore an excellent primary standard.
Mohr’s Method:
Principle
A German chemist - Karl Friedrich Mohr devised this method in 1856
The method involves direct titration.
titrant -Silver nitrate
an analyte/Sample/Tirand -Halides (chloride ion) solution is used
Indicator- Potassium Chromate [chromate ions (CrO42-)] as the indicator
For the argentometric determination of bromide, chloride and cyanide
ions. Estimation is carried out – in neutral medium](https://image.slidesharecdn.com/precipitationtitrations-d-240718060305-46470f48/75/Precipitation-titrations-D-Pharma-pptx-6-2048.jpg)