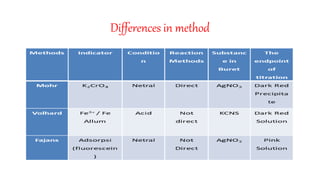
This document describes different methods of argentometric titration:
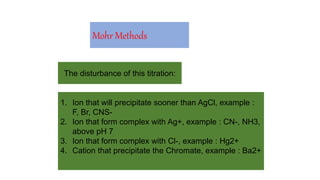
1. Mohr's method uses silver nitrate solution and potassium chromate indicator to determine the content of chloride and bromide ions through a precipitation reaction.
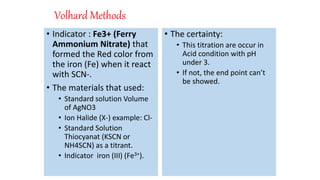
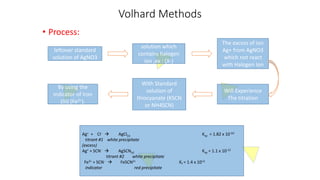
2. Volhard's method uses thiocyanate ion as the titrant and ferric ion indicator to titrate excess silver ions from the initial reaction of silver nitrate with halide ions.
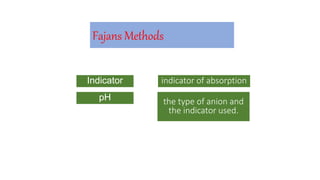
3. Fajans' method uses a pH indicator absorption method to determine the type of anion present based on the indicator used.