This document provides an overview of key concepts in general chemistry including:
1. The physical and chemical properties of matter, the different physical states of substances, and examples of physical and chemical changes.
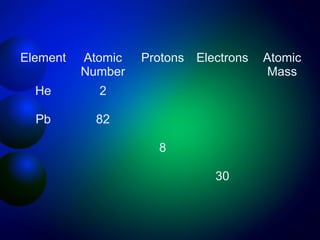
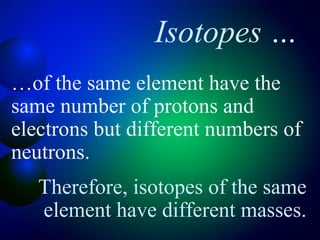
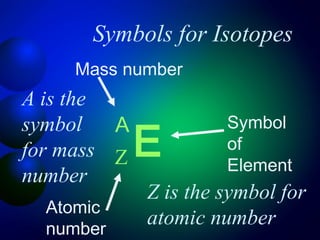
2. The structure of atoms including protons, neutrons, electrons, and isotopes. Key atomic structure concepts like atomic number and mass number are introduced.
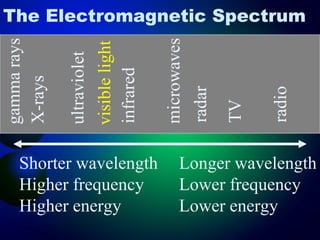
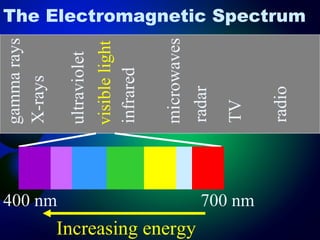
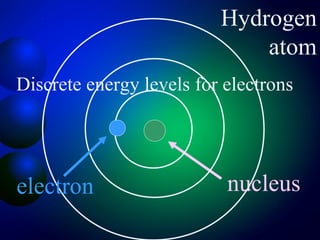
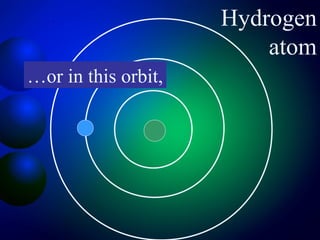
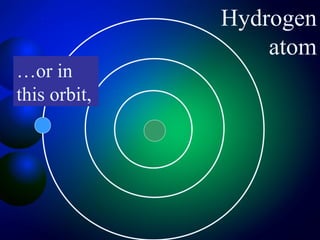
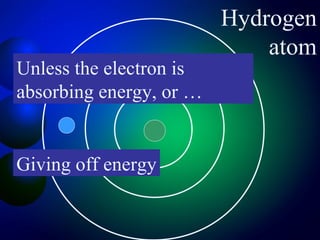
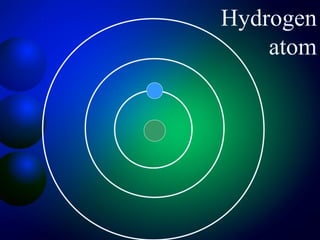
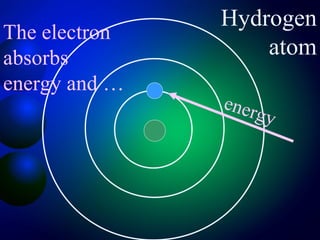
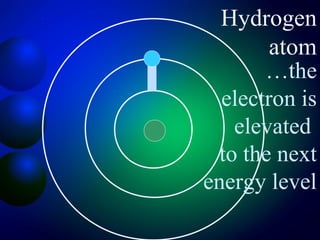
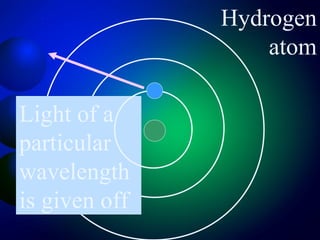
3. Energy levels of electrons in atoms, specifically the Bohr model of the hydrogen atom. Light is emitted when electrons change energy levels.
4. The quantum mechanical model which describes electrons existing as waves around the nucleus at different energy levels rather than classical orbits.