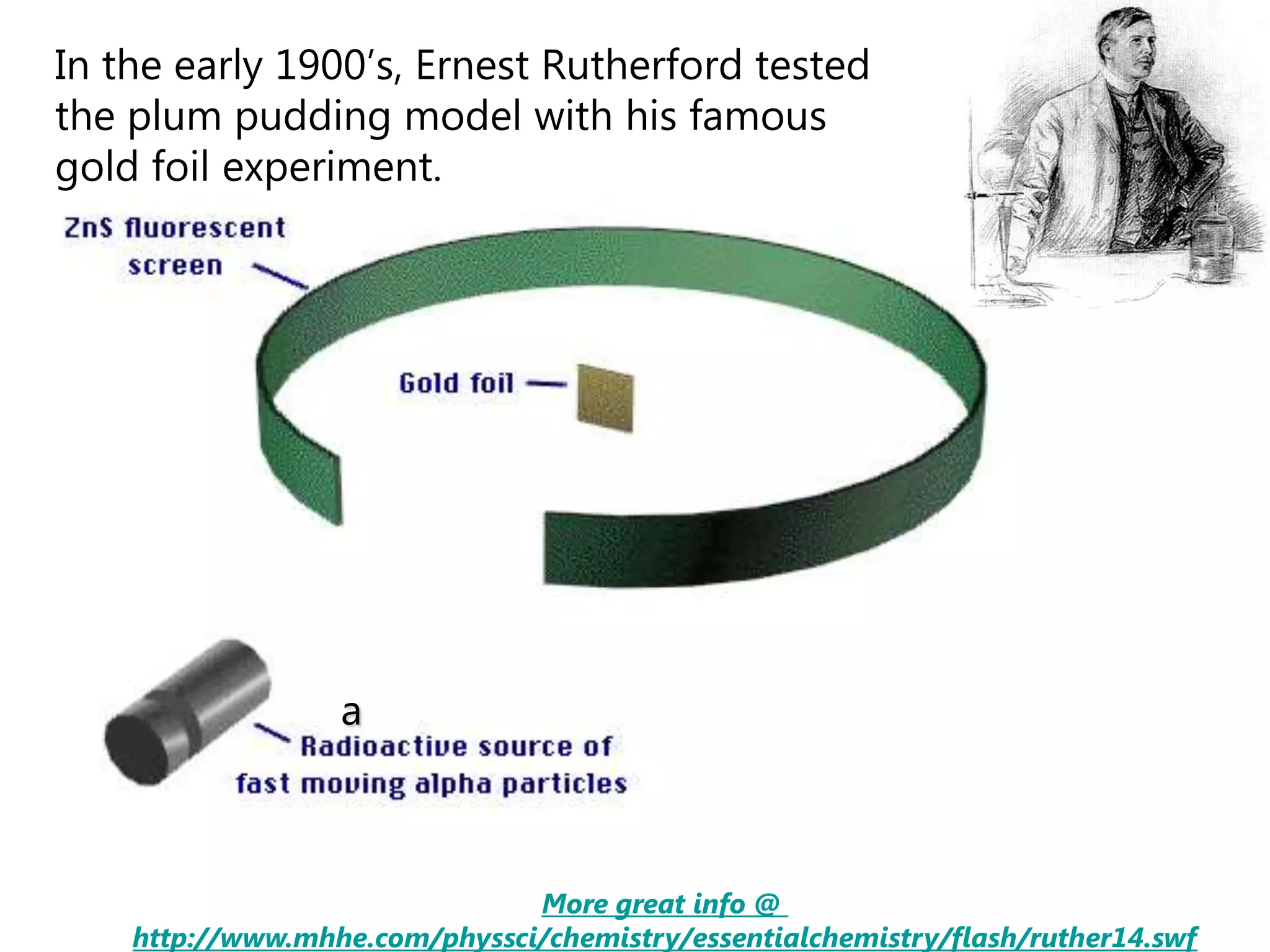
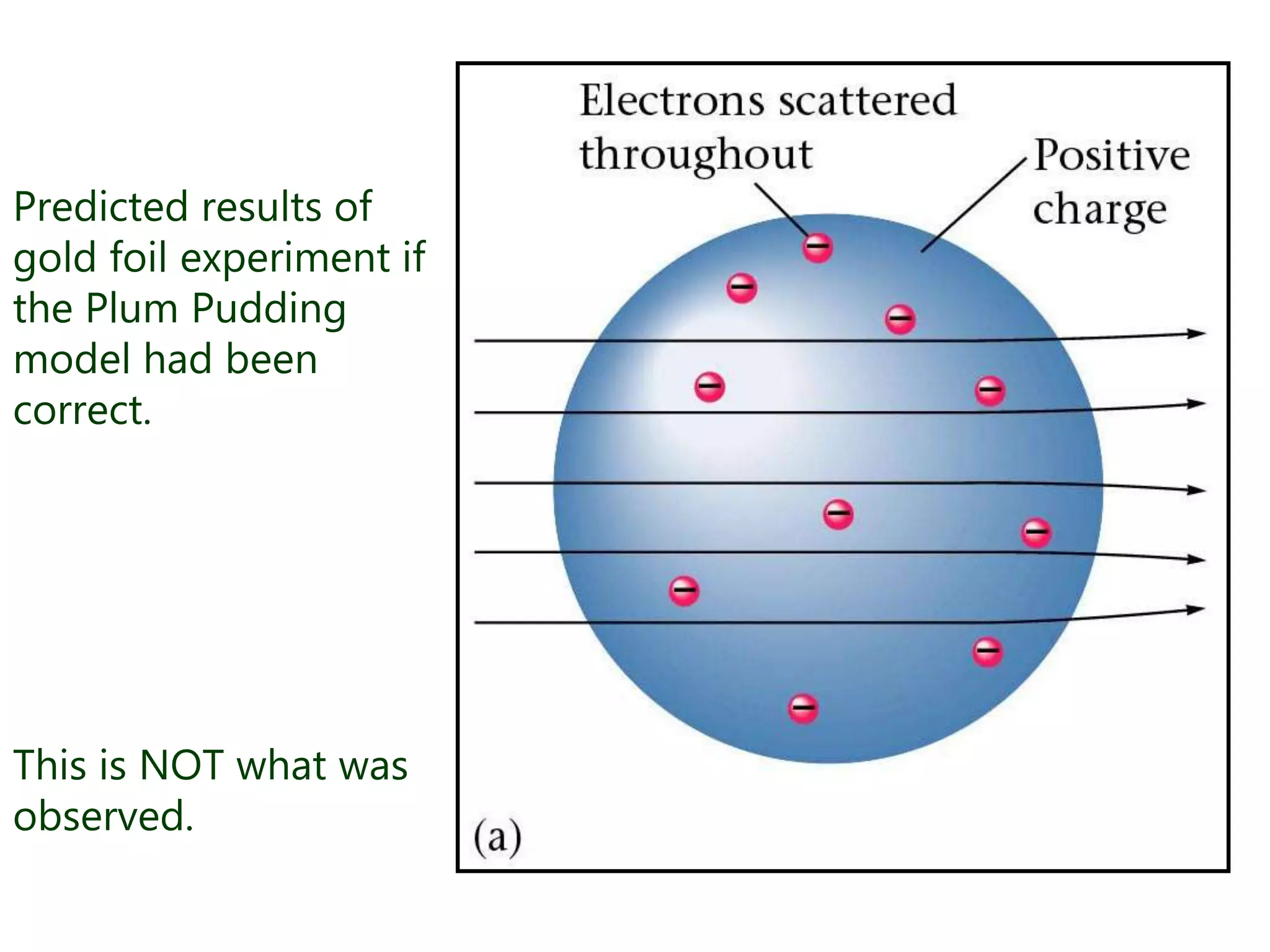
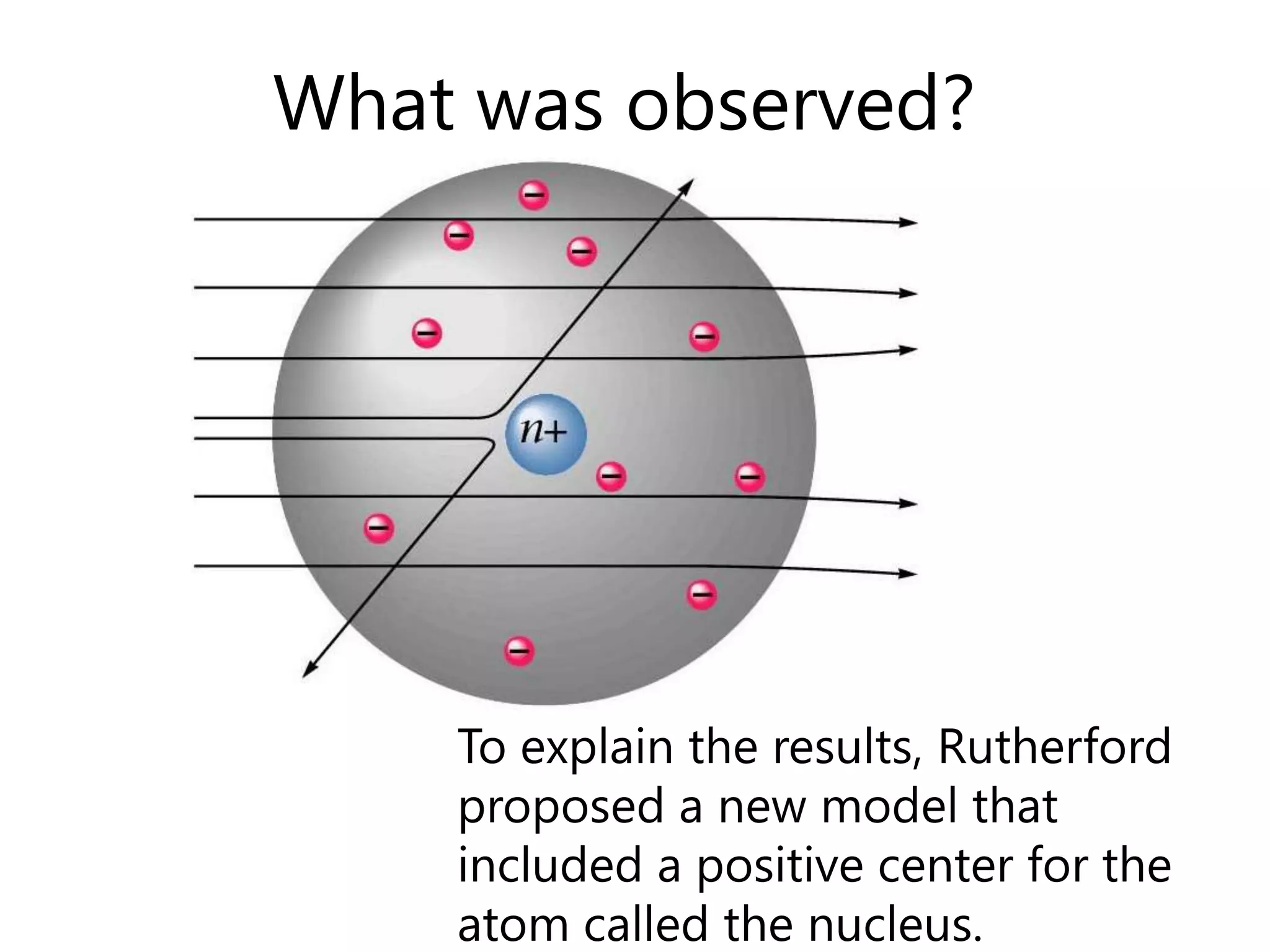
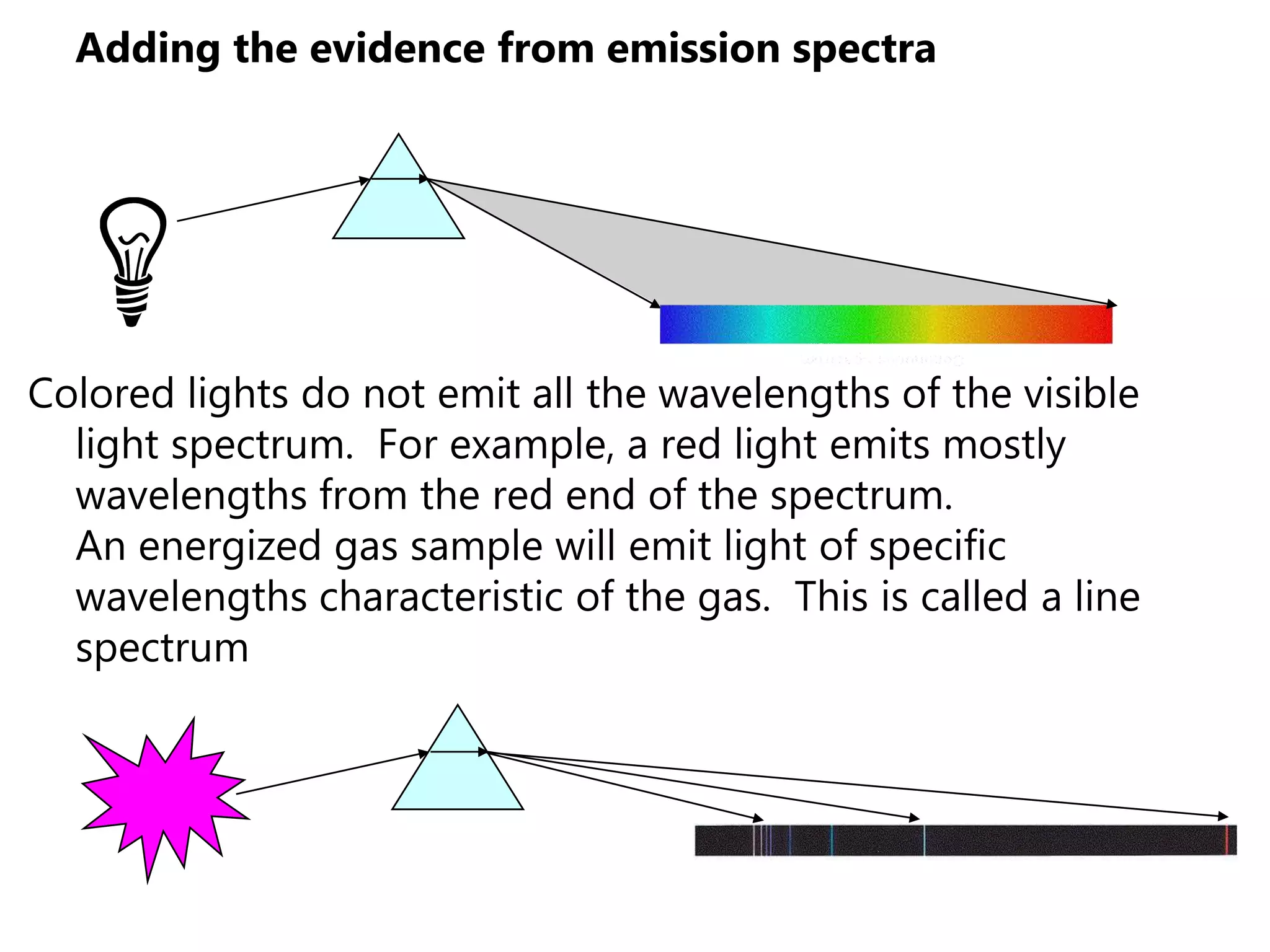
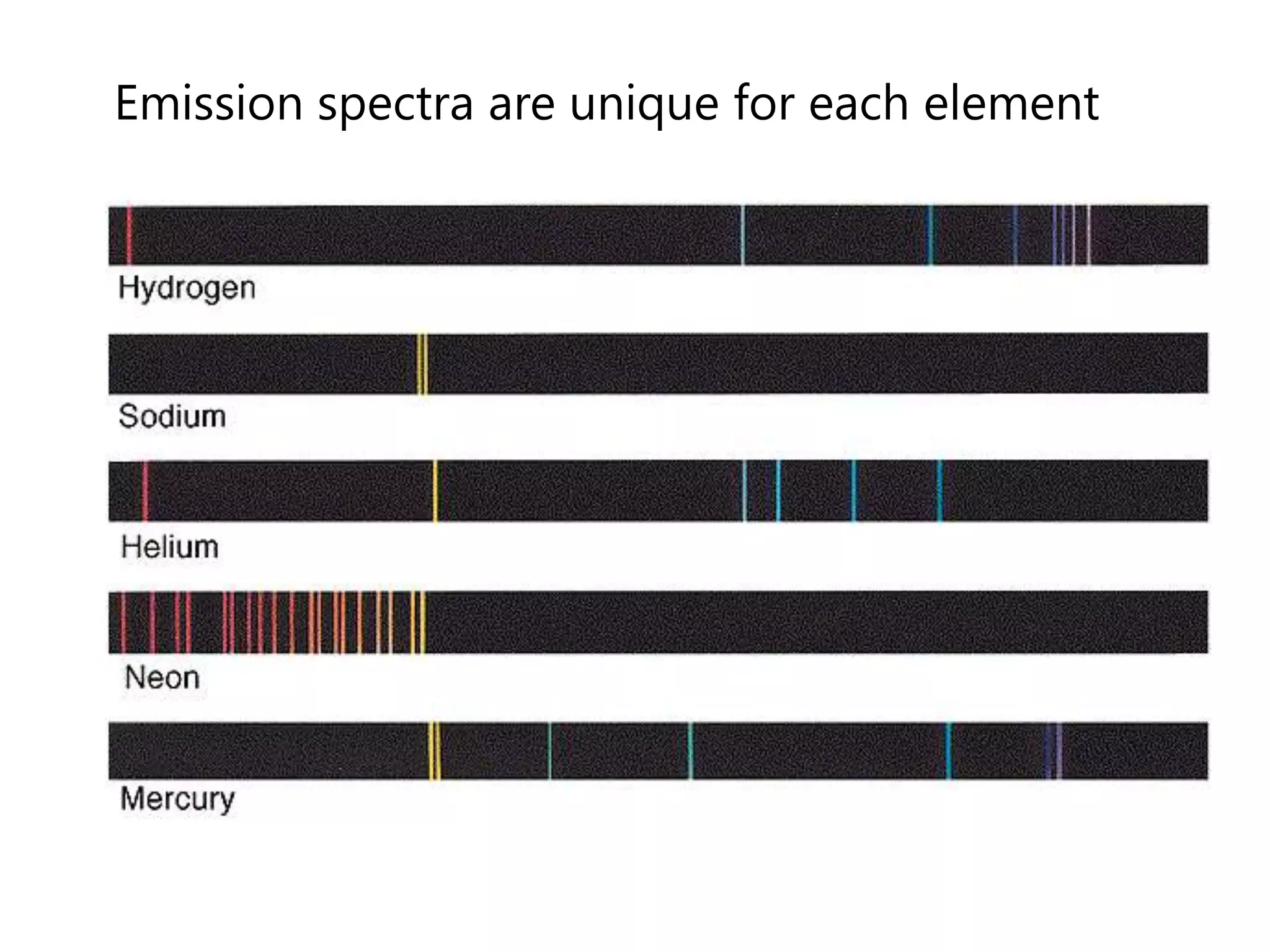
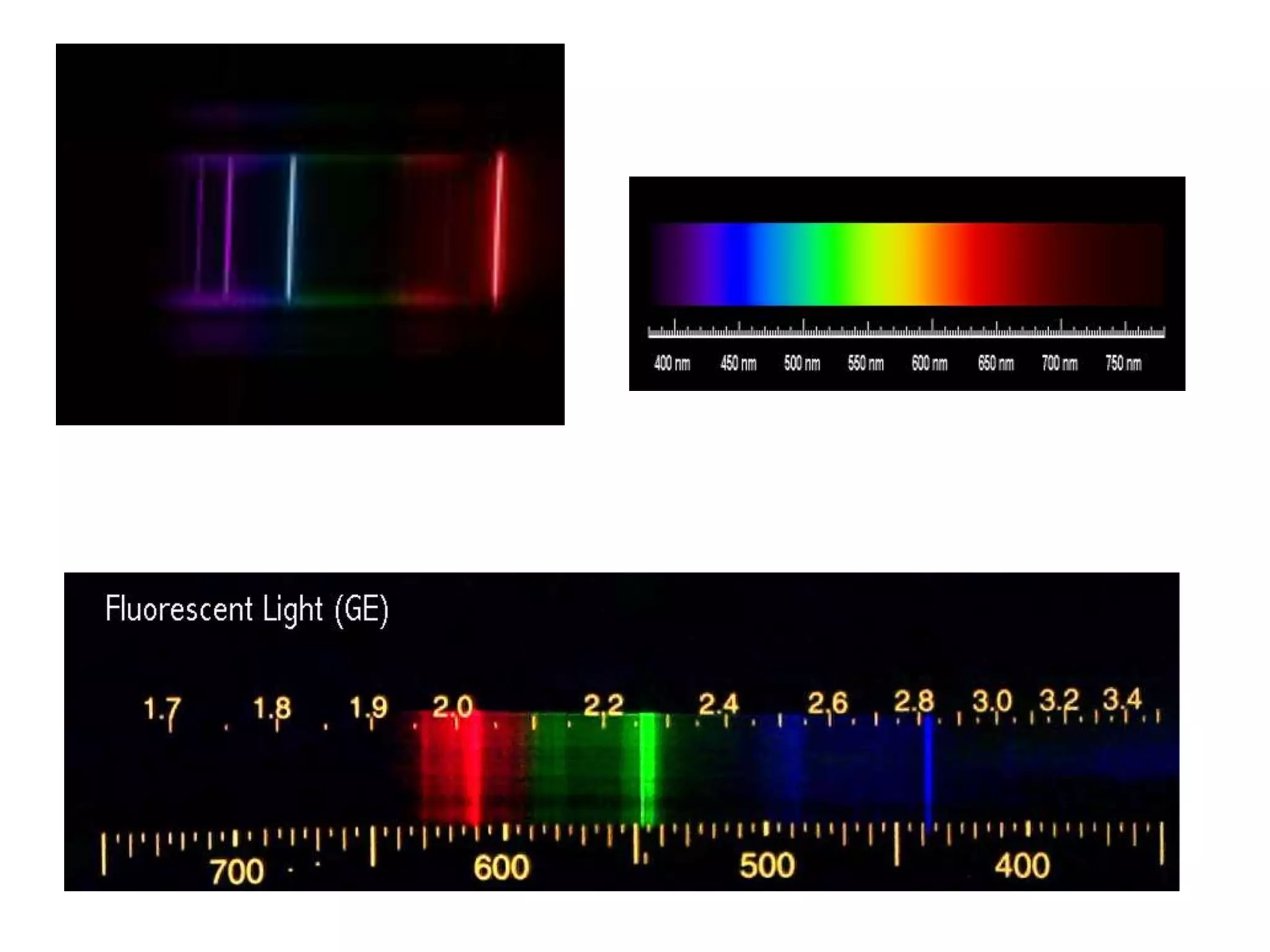
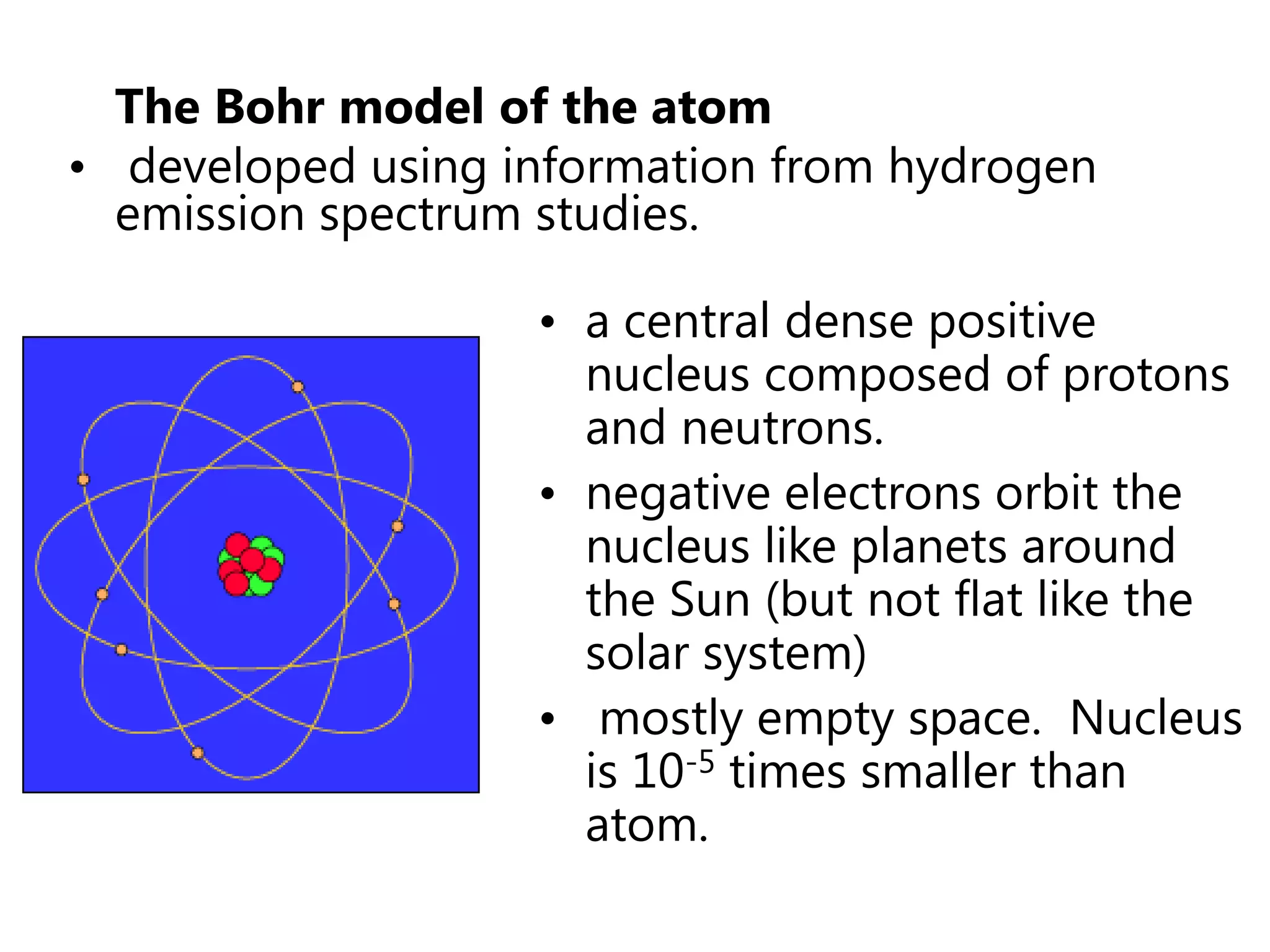
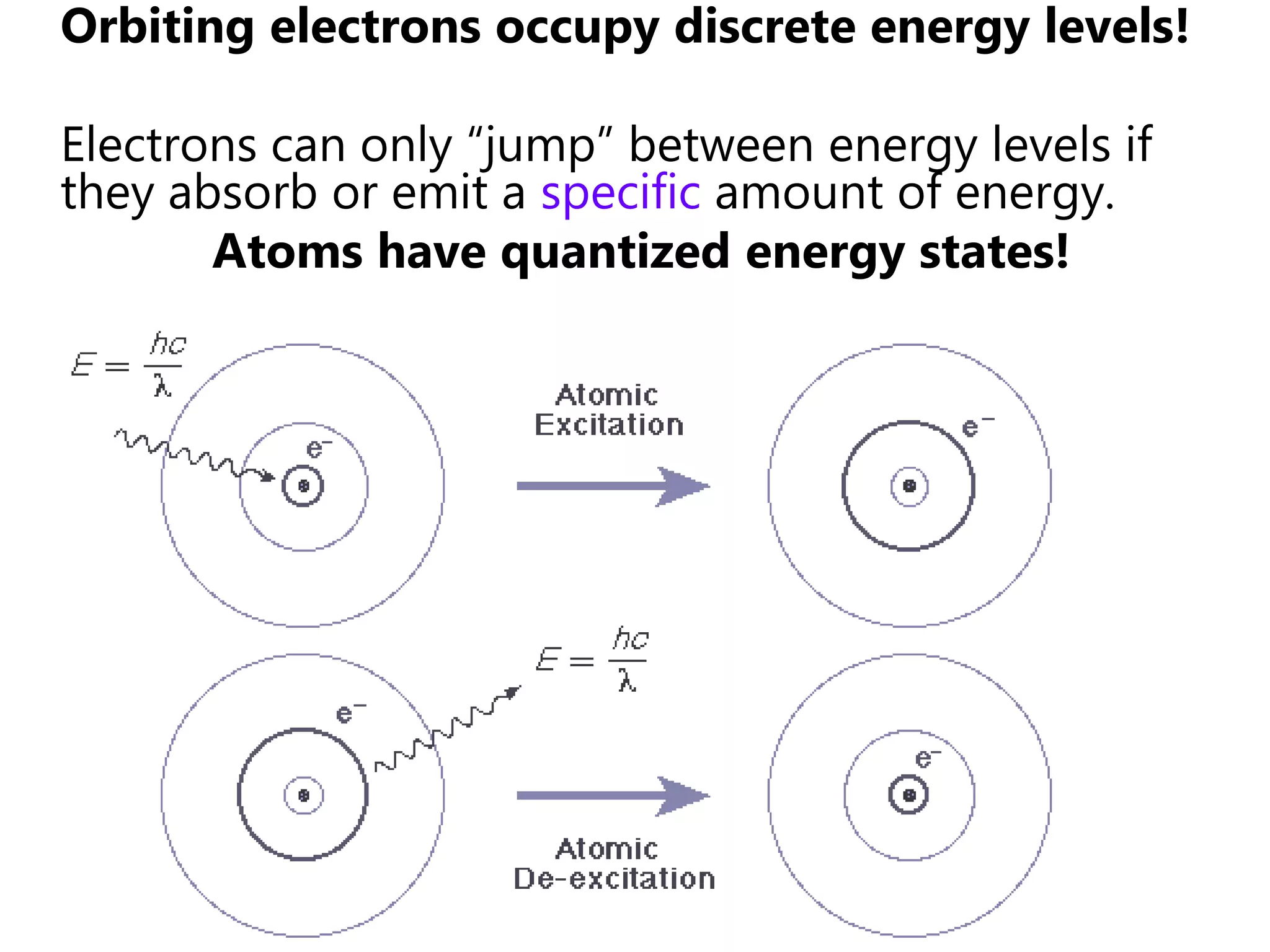
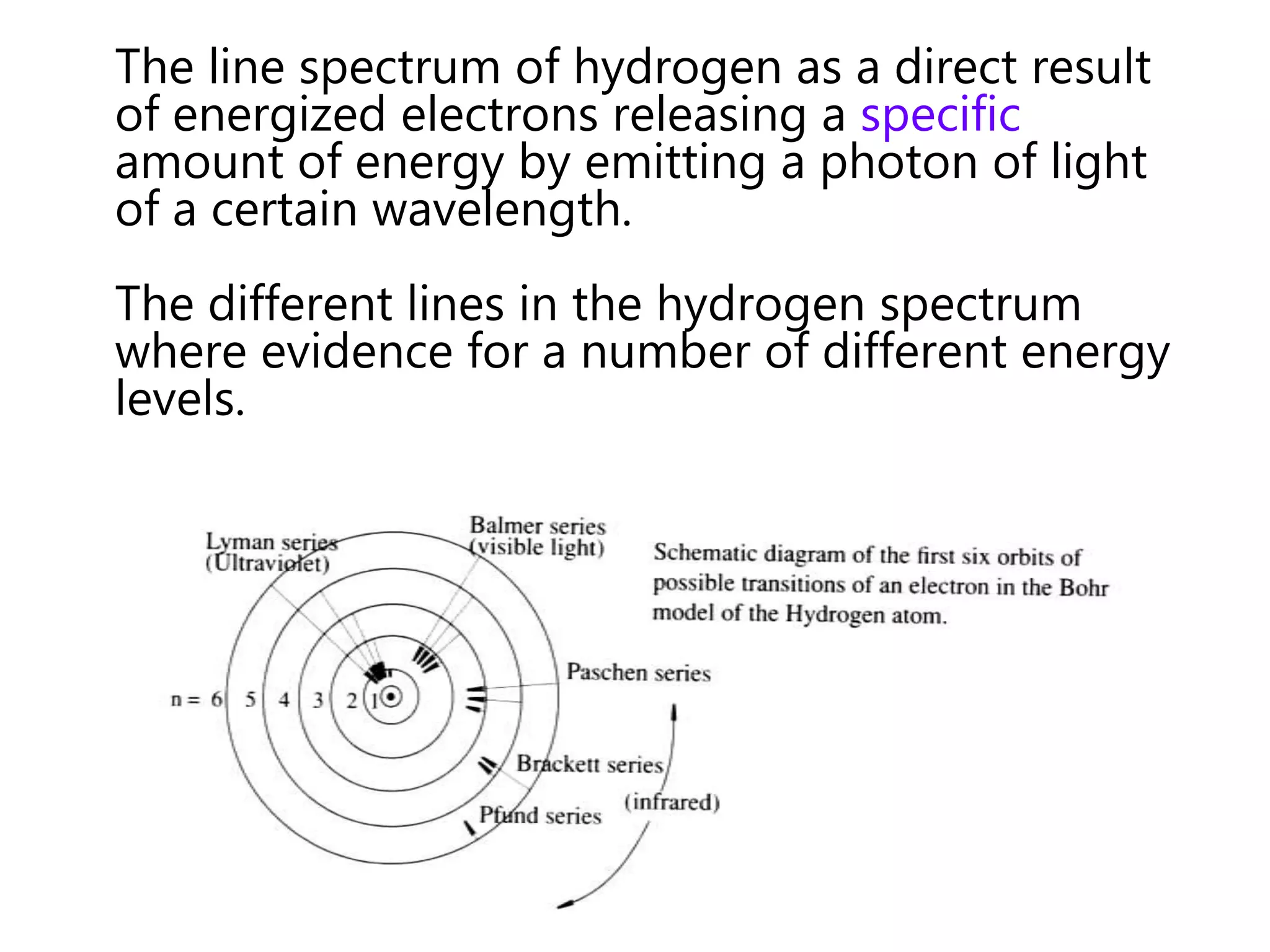
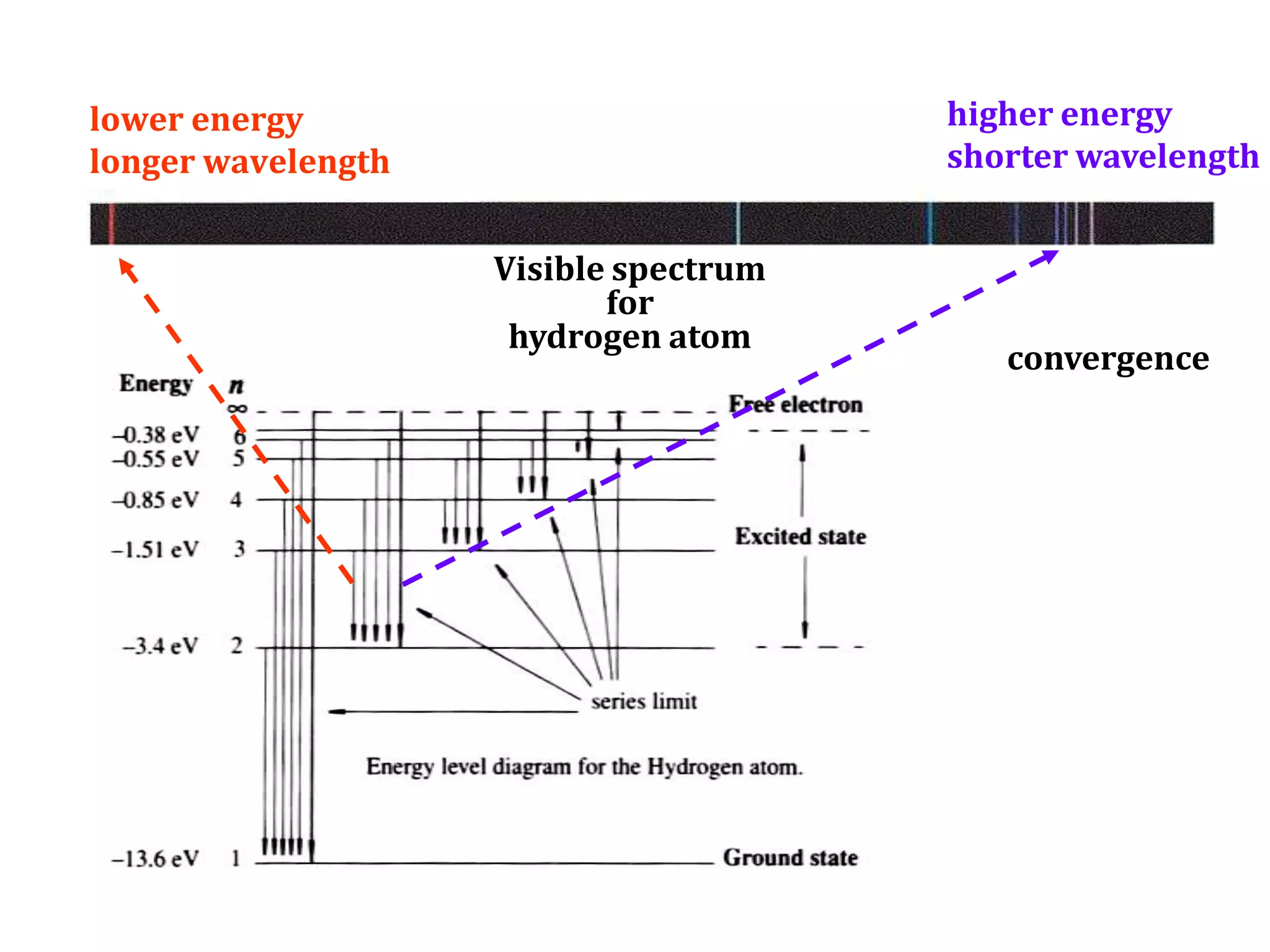
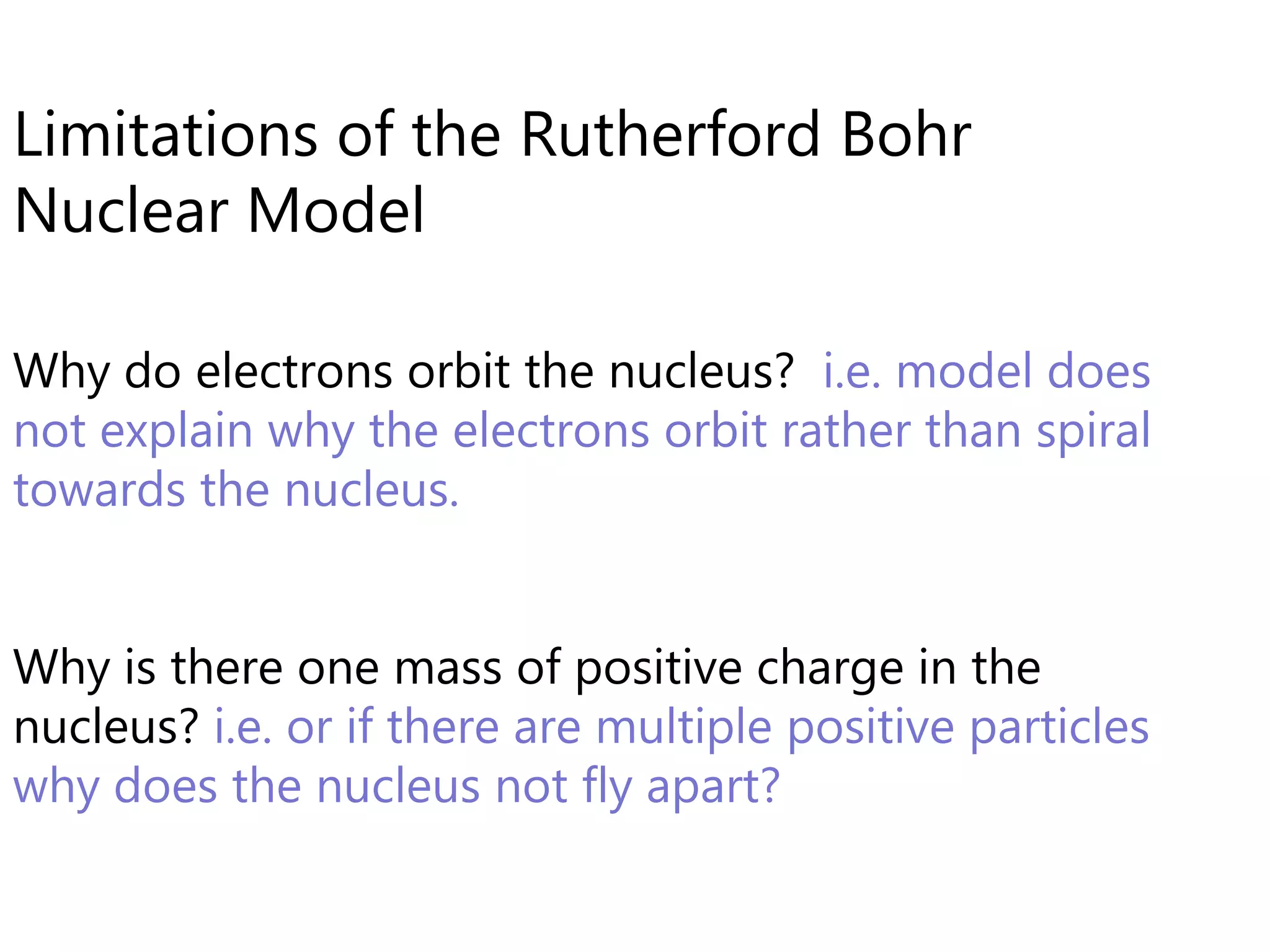
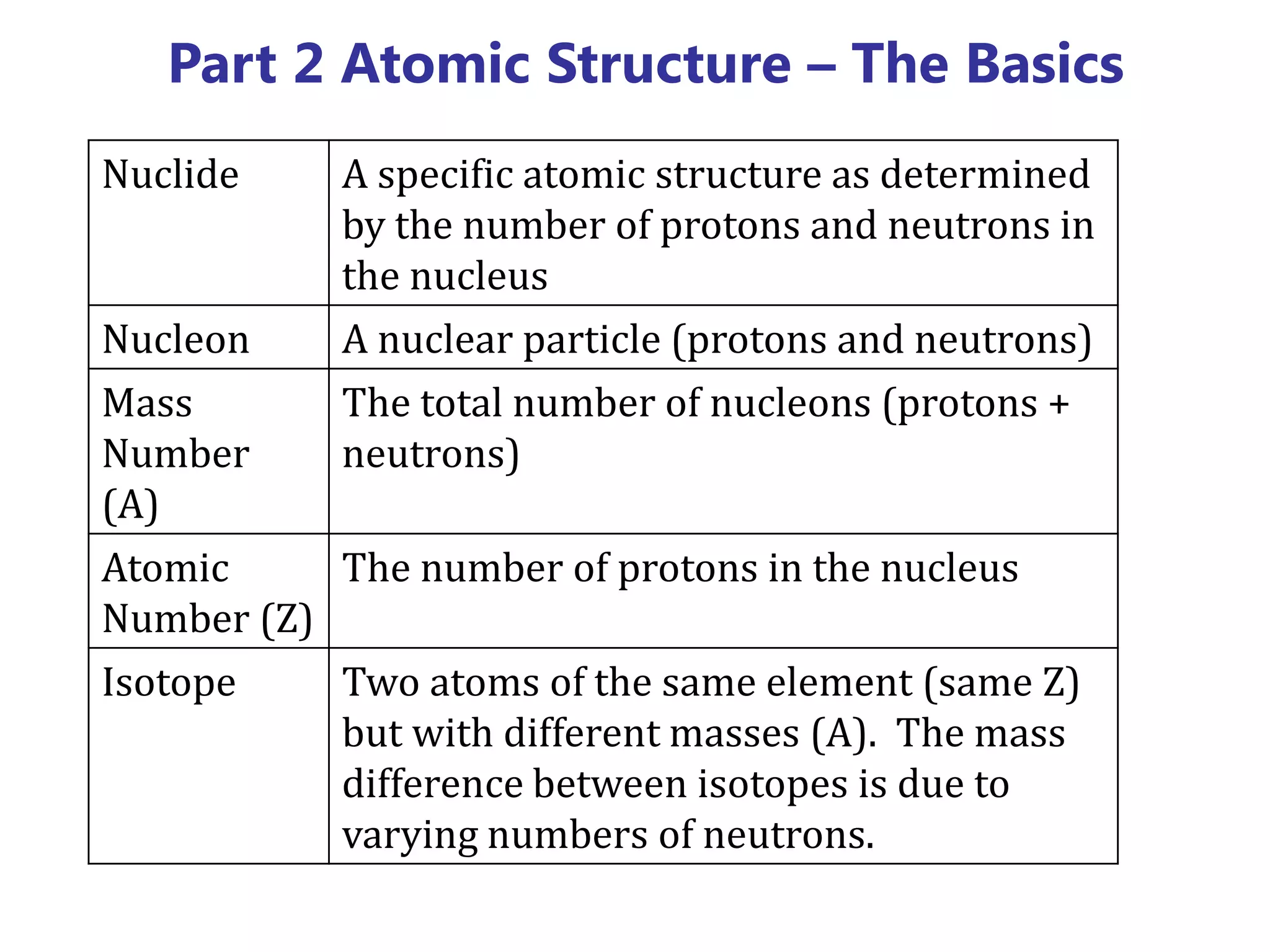
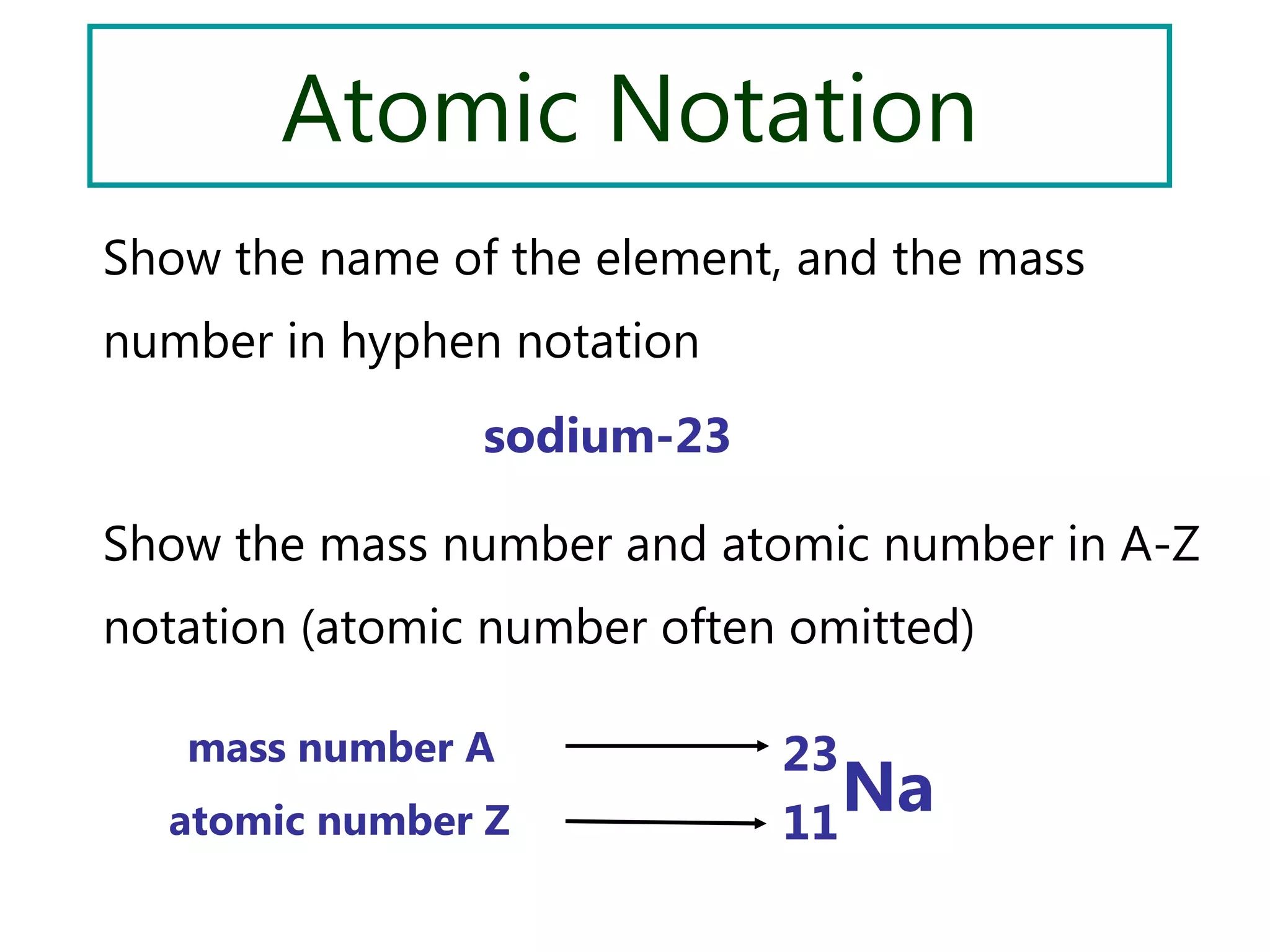
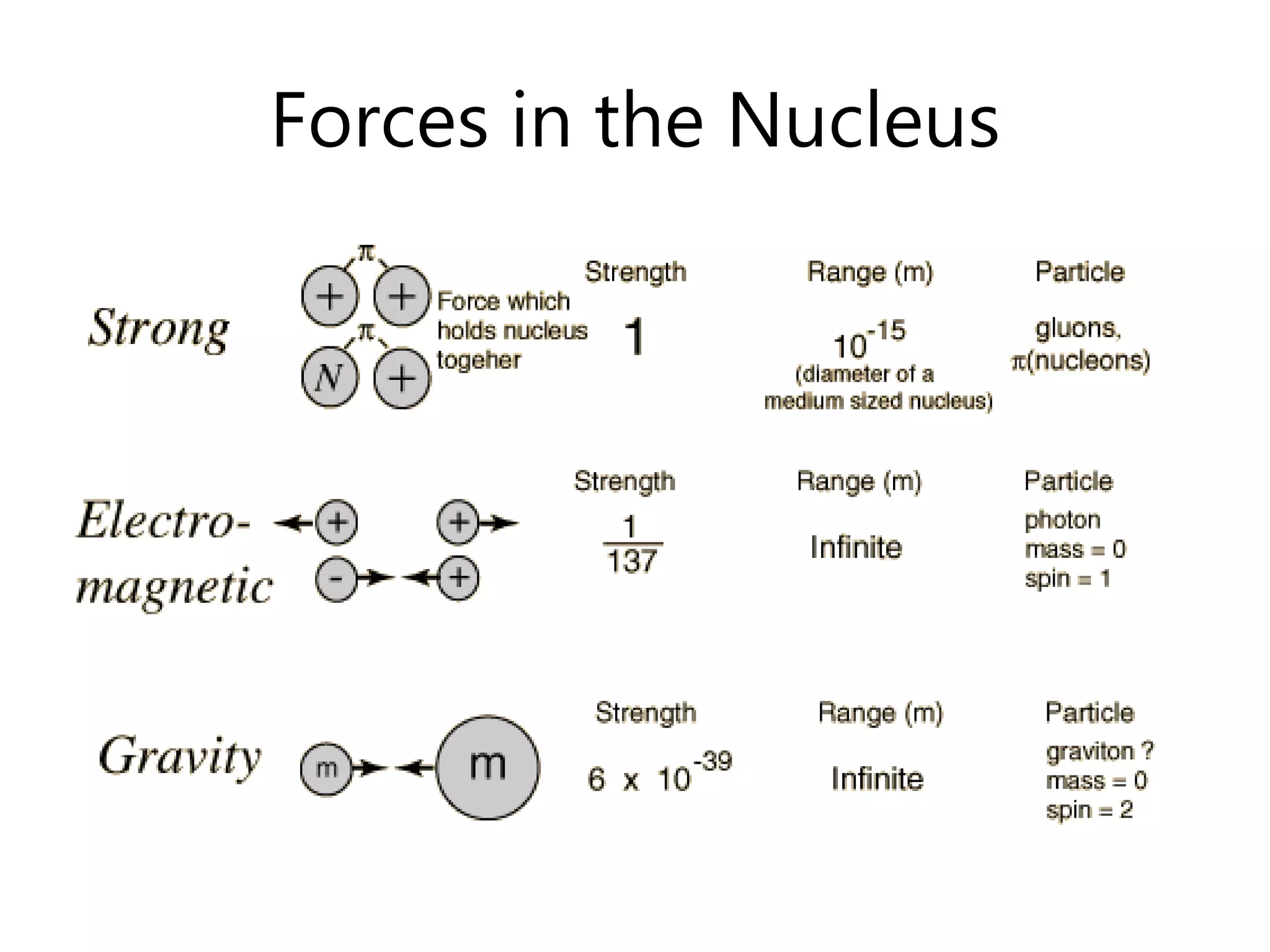
This document discusses the development of atomic models from ancient Greek ideas to Rutherford's gold foil experiment and Bohr's model of the hydrogen atom. It introduces Dalton's atomic theory and developments like the plum pudding model, discovery of the electron, Rutherford's nuclear model, and Bohr's explanation of emission spectra. Key concepts covered include atomic structure, isotopes, atomic notation, and forces that hold the nucleus together.