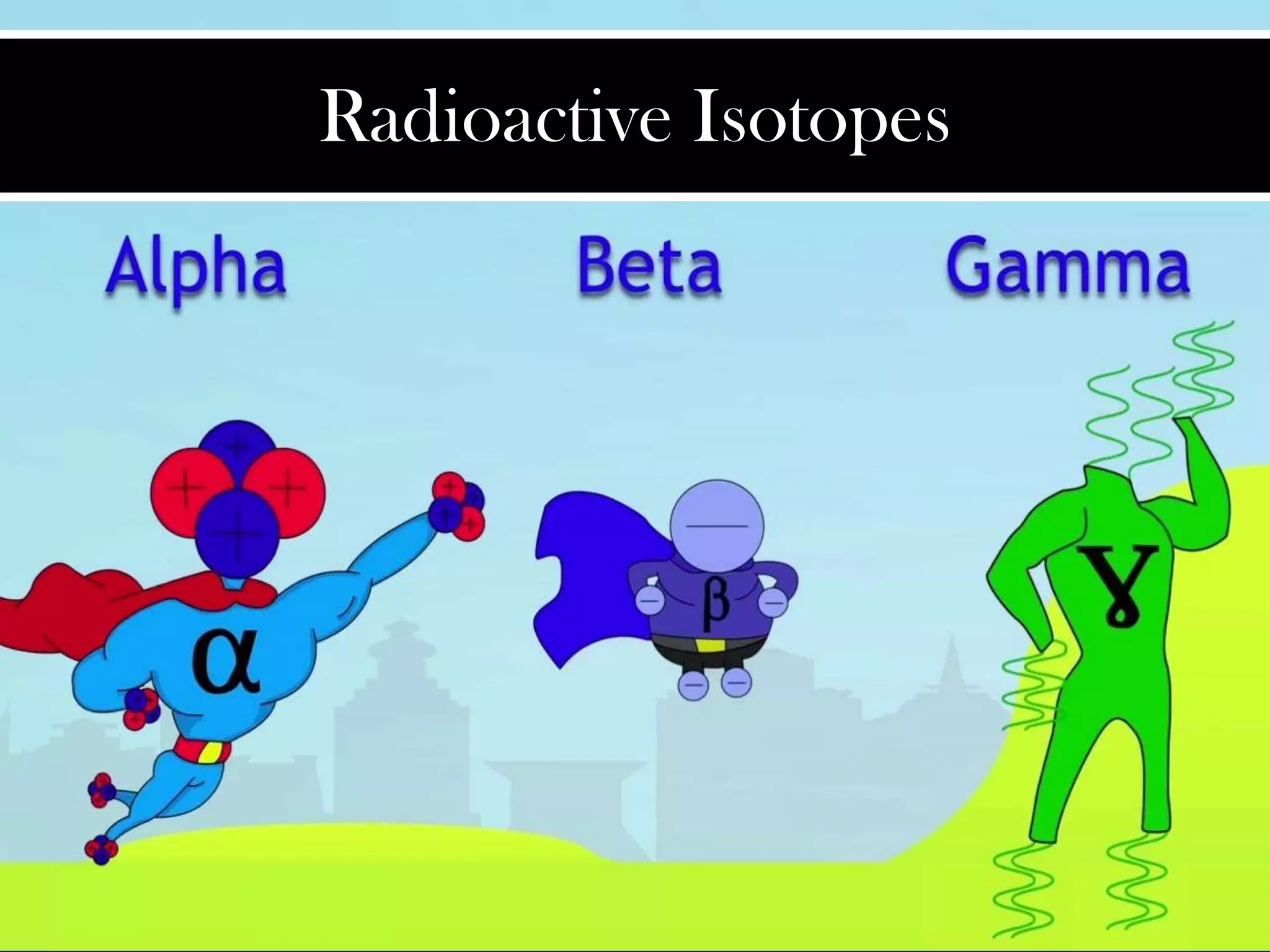
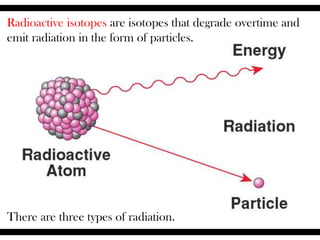
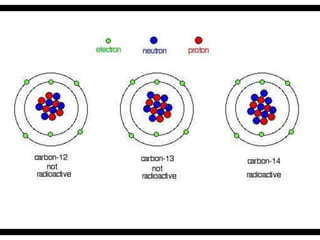
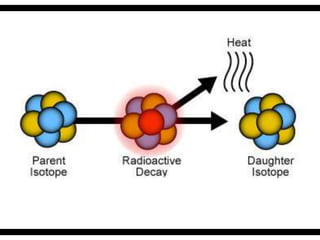
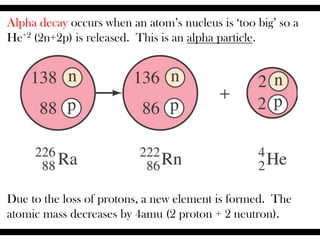
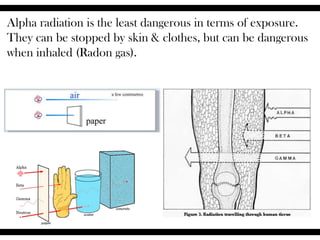
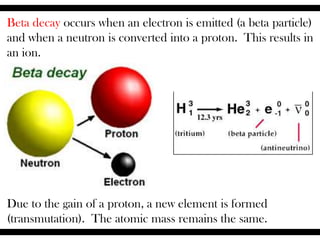
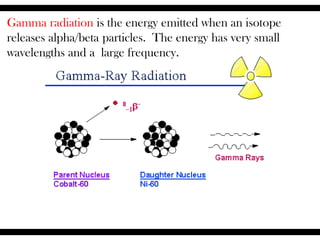
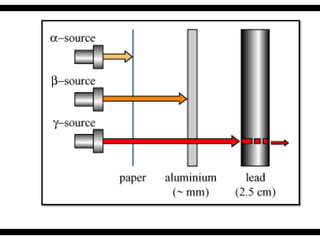
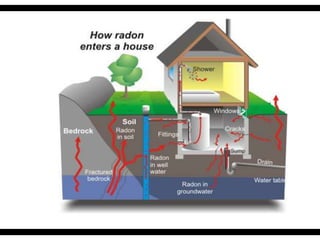
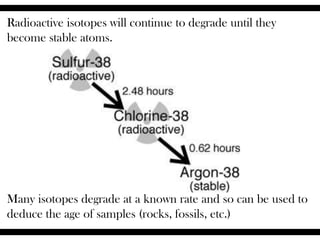
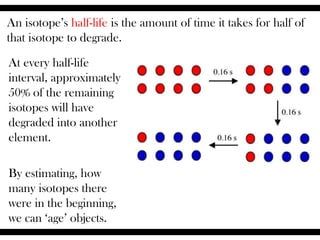
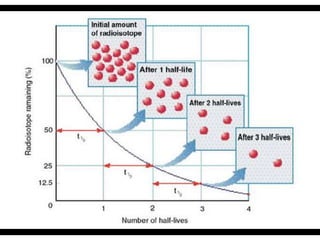
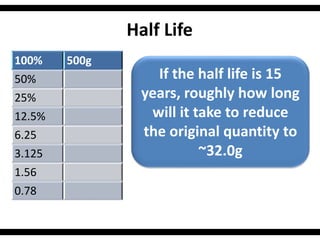
Radioactive isotopes emit radiation as they decay over time. There are three main types of radiation: alpha, beta, and gamma. Alpha radiation occurs when an atom emits an alpha particle, which is a helium nucleus, changing the emitting atom into a new element. Beta radiation occurs when a neutron converts to a proton, emitting an electron and creating an ion. Gamma radiation is high energy electromagnetic radiation emitted during alpha and beta decay. Radioactive isotopes will continue decaying until they reach a stable form, and their half-life can be used to determine the age of samples by estimating their original quantity.