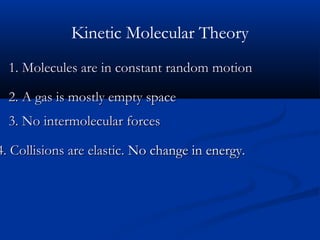
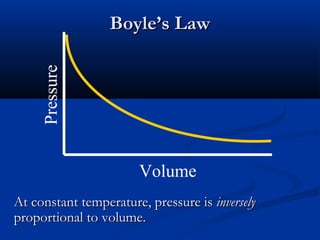
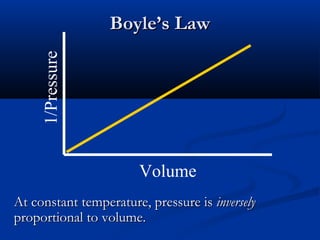
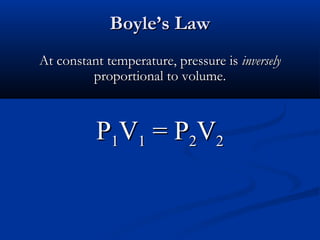
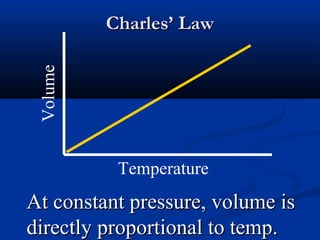
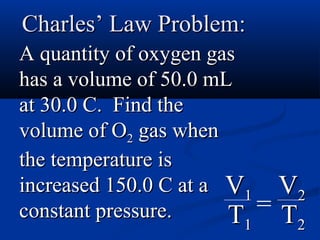
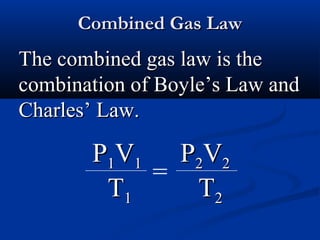
Gases expand to fill their container, take the shape of the container, and are highly compressible. Gases have low densities and mix uniformly. According to the kinetic molecular theory, gas molecules are in constant random motion with mostly empty space between them and no intermolecular forces. Boyle's law states that at constant temperature, pressure and volume are inversely proportional. Charles' law says that at constant pressure, volume is directly proportional to temperature. The combined gas law incorporates both Boyle's and Charles' laws.