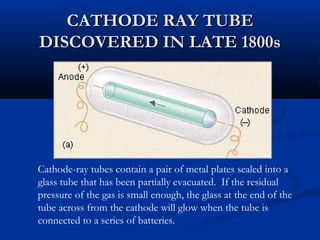
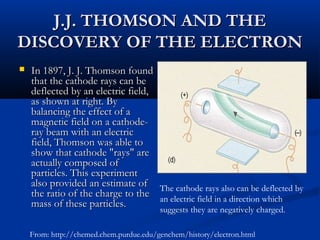
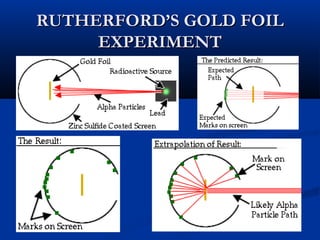
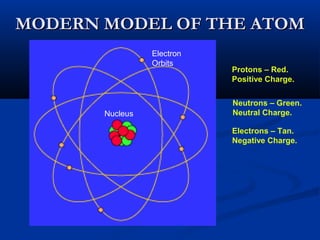
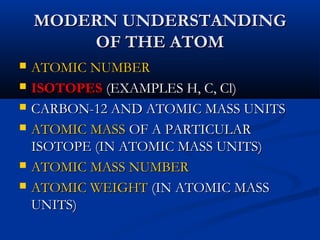
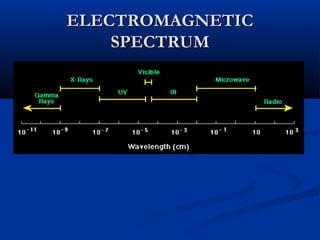
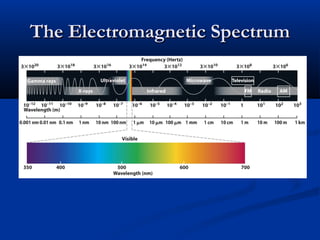
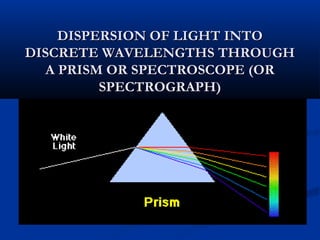
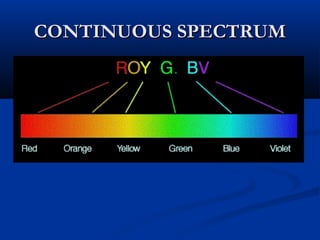
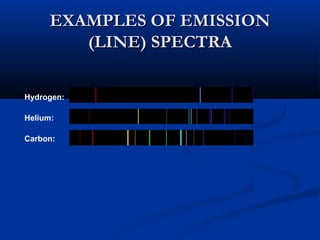
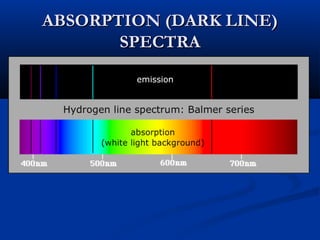
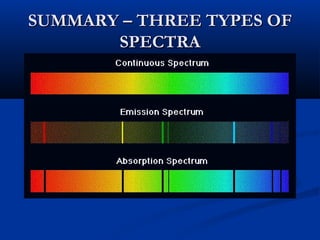
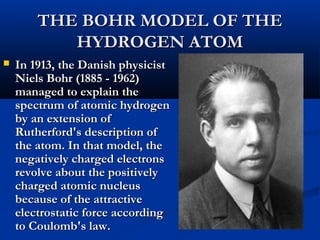
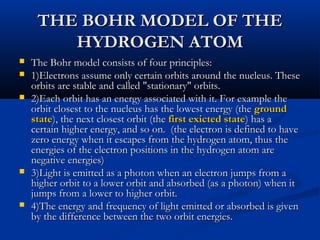
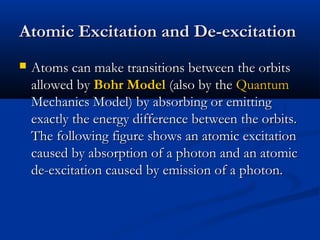
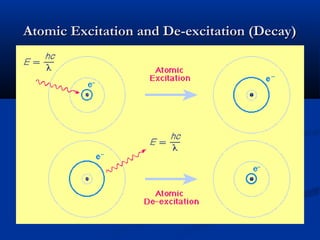
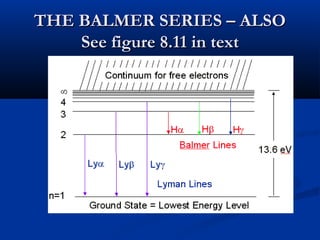
Democritus first proposed the idea of atoms in 400 BC as the smallest indivisible particles of matter. In the early 1800s, John Dalton developed the first modern atomic theory, proposing that all matter is made of atoms that are identical for a given element. In the late 1800s and early 1900s, scientists like J.J. Thomson, Ernest Rutherford, and others discovered subatomic particles like electrons and the nucleus through experiments such as cathode ray tubes and bombarding atoms with alpha particles. These discoveries led to the modern understanding of atoms as a dense nucleus of protons and neutrons surrounded by electrons in orbits.