Embed presentation
Downloaded 97 times



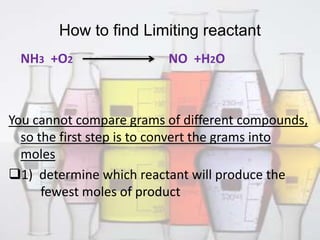


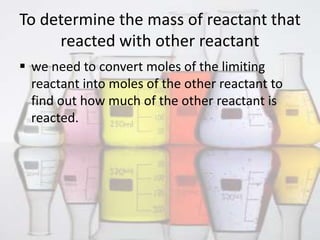

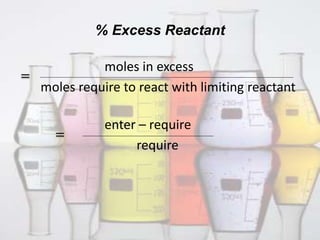
This document discusses limiting and excess reactants in chemical reactions. It defines a limiting reactant as the reactant that is consumed first or ends the reaction. It provides two methods to determine the limiting reactant: 1) converting reactants to moles and seeing which produces the fewest moles of product, and 2) comparing reactant ratios in the chemical equation and actual amounts. The excess reactant is any reactant other than the limiting one that remains after the reaction. It defines percent excess reactant as the moles in excess divided by the moles required to react with the limiting reactant.








