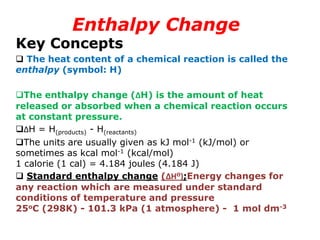
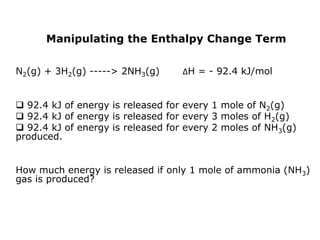
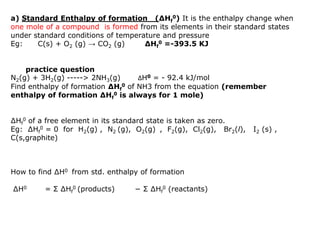
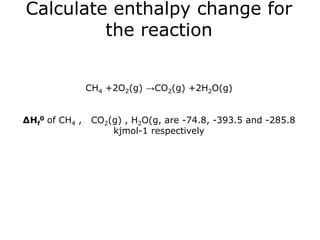
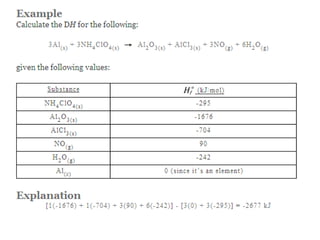
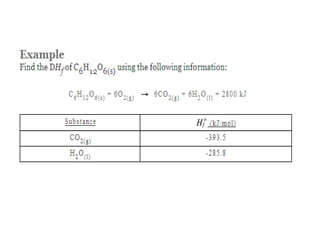
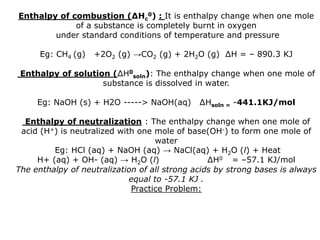
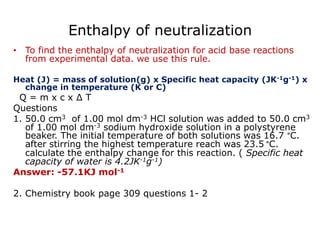
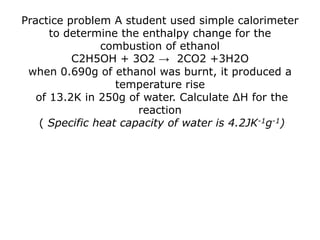
The document discusses enthalpy change (ΔH) which is the amount of heat released or absorbed during a chemical reaction under constant pressure. ΔH is calculated as the enthalpy of products minus reactants. Standard enthalpy change (ΔH°) is measured under standard temperature and pressure conditions. Some key enthalpy terms discussed include standard enthalpy of formation (ΔHf°), enthalpy of combustion (ΔHc°), enthalpy of solution (ΔHsoln), and enthalpy of neutralization. Practice problems are provided to calculate enthalpy changes using experimental heat and temperature change data.