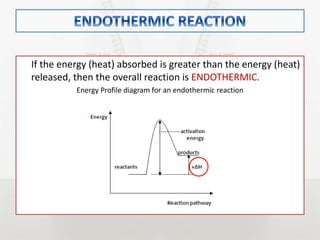
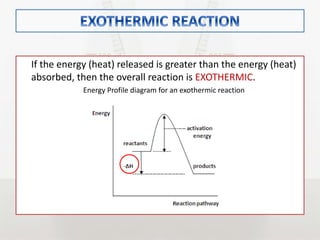
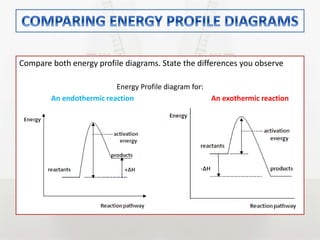
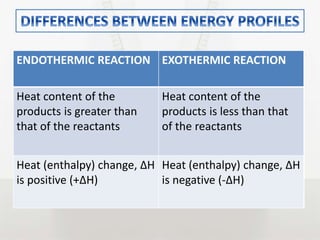
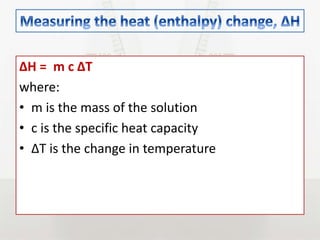
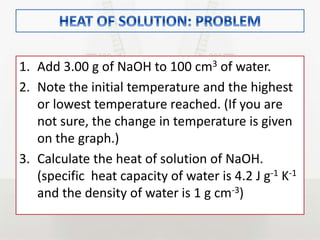
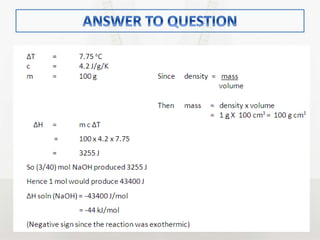
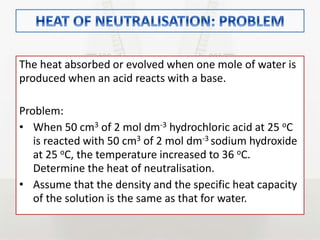
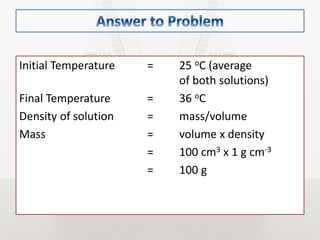
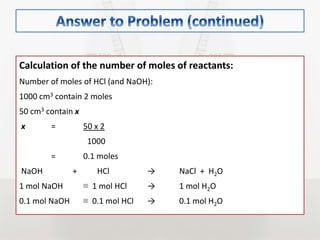
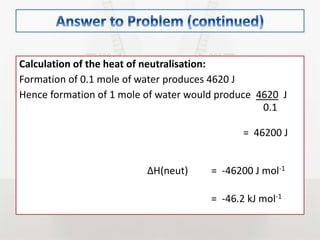
This document discusses energy changes that occur during chemical reactions. It defines endothermic and exothermic reactions, and explains how to interpret energy diagrams for these reactions. Key terms like activation energy, enthalpy change, heat of solution, and heat of neutralization are defined. The document provides examples of how to calculate heat of solution and heat of neutralization using experimental data and equations.