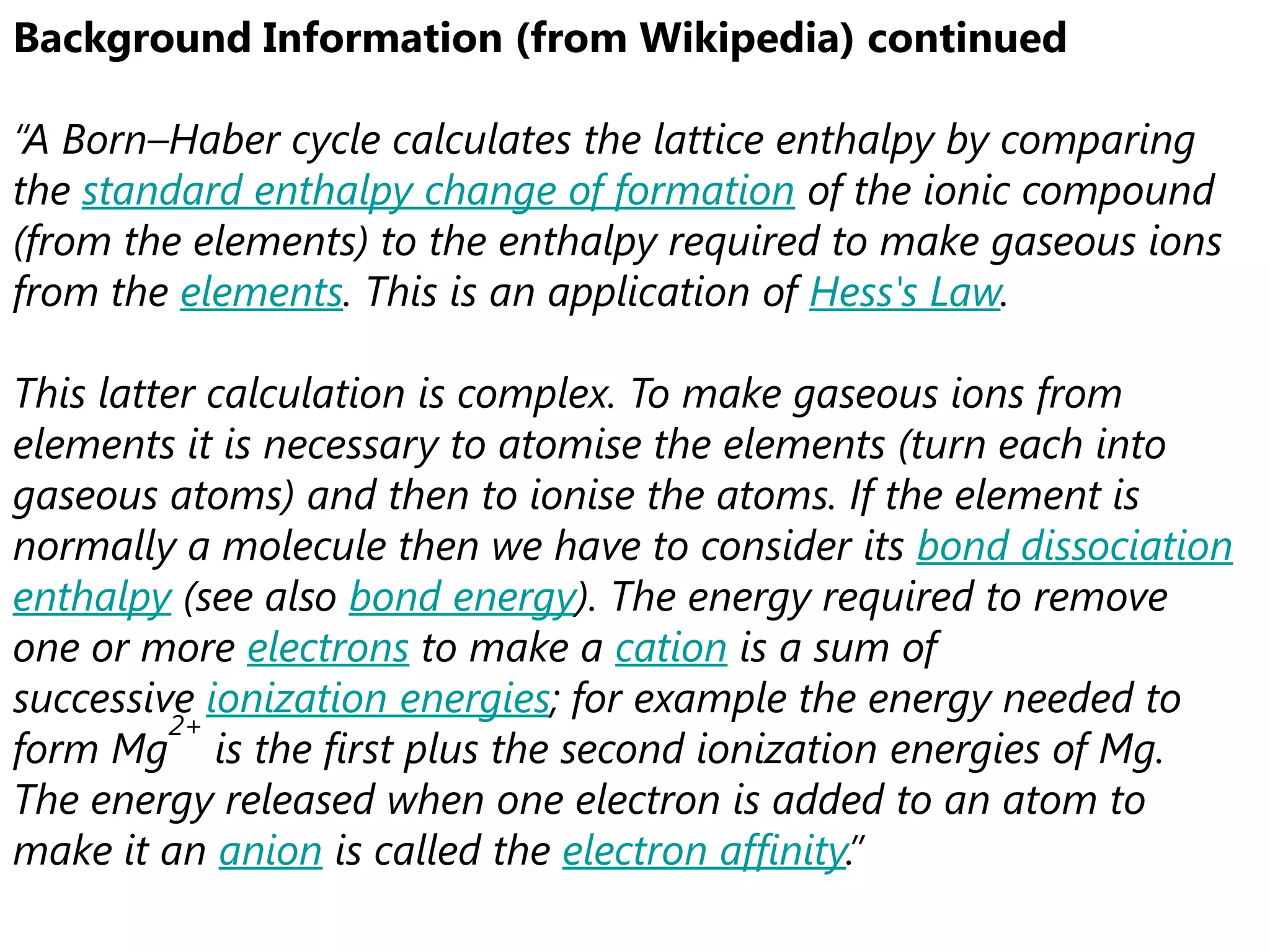
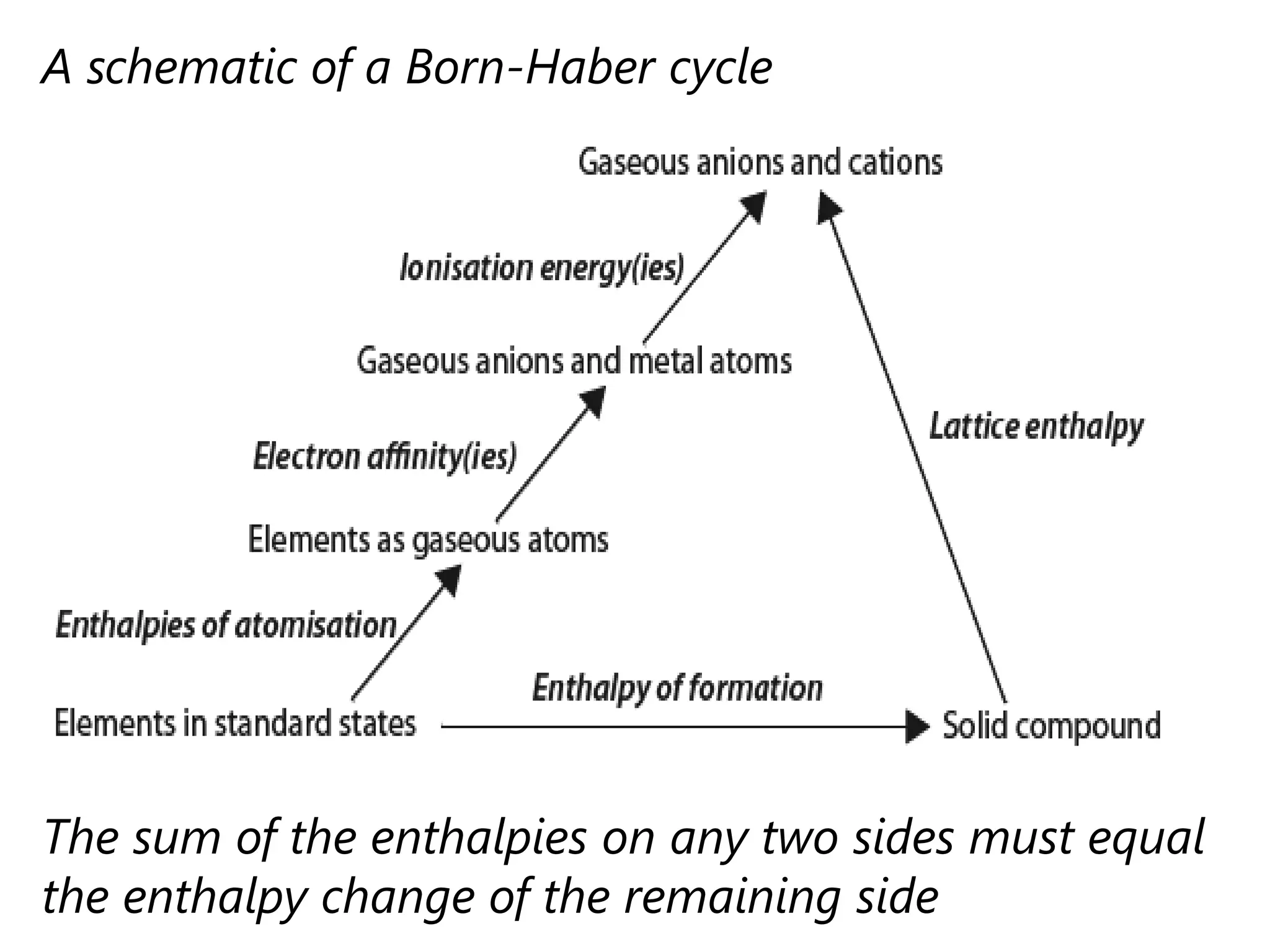
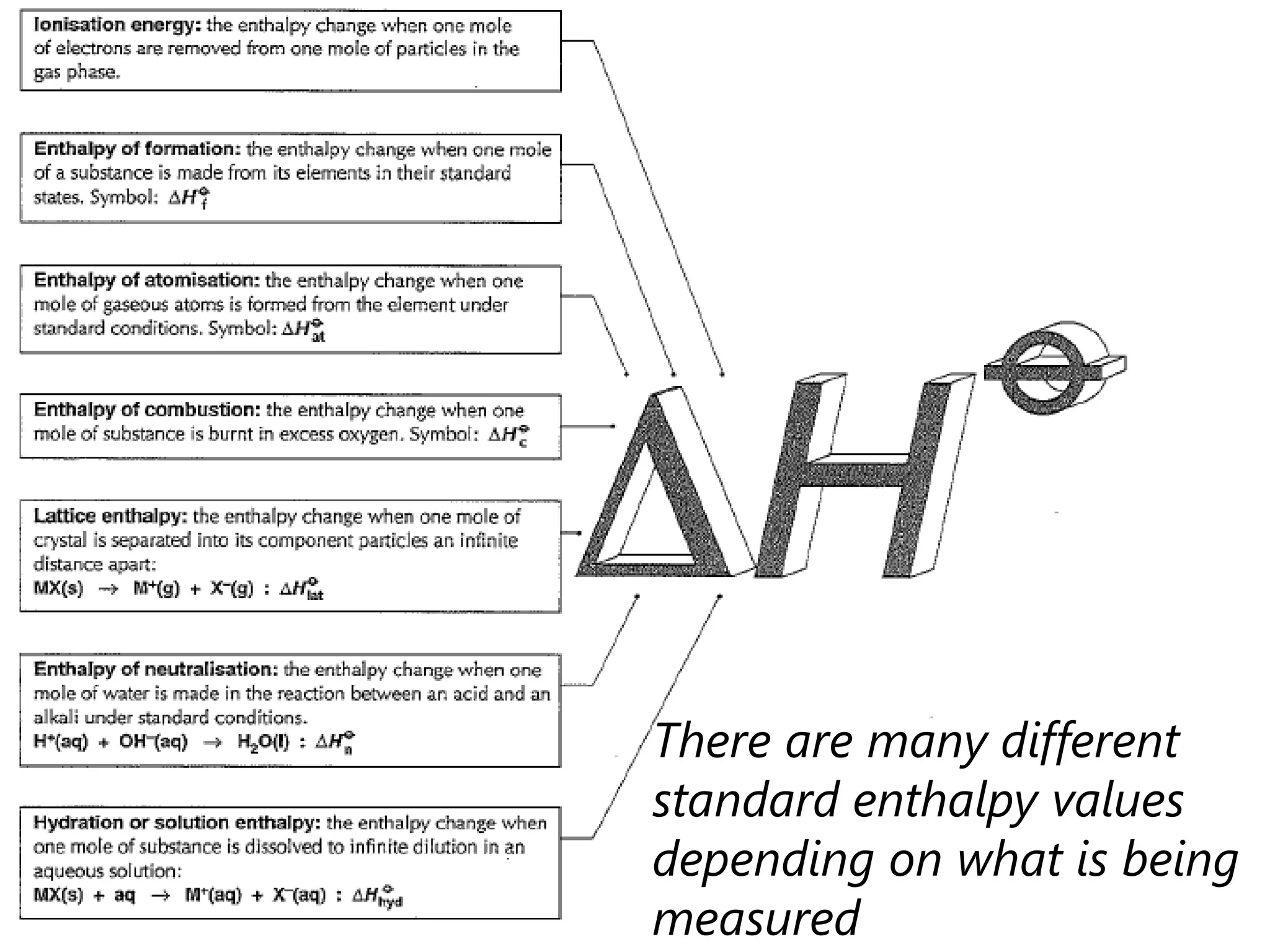
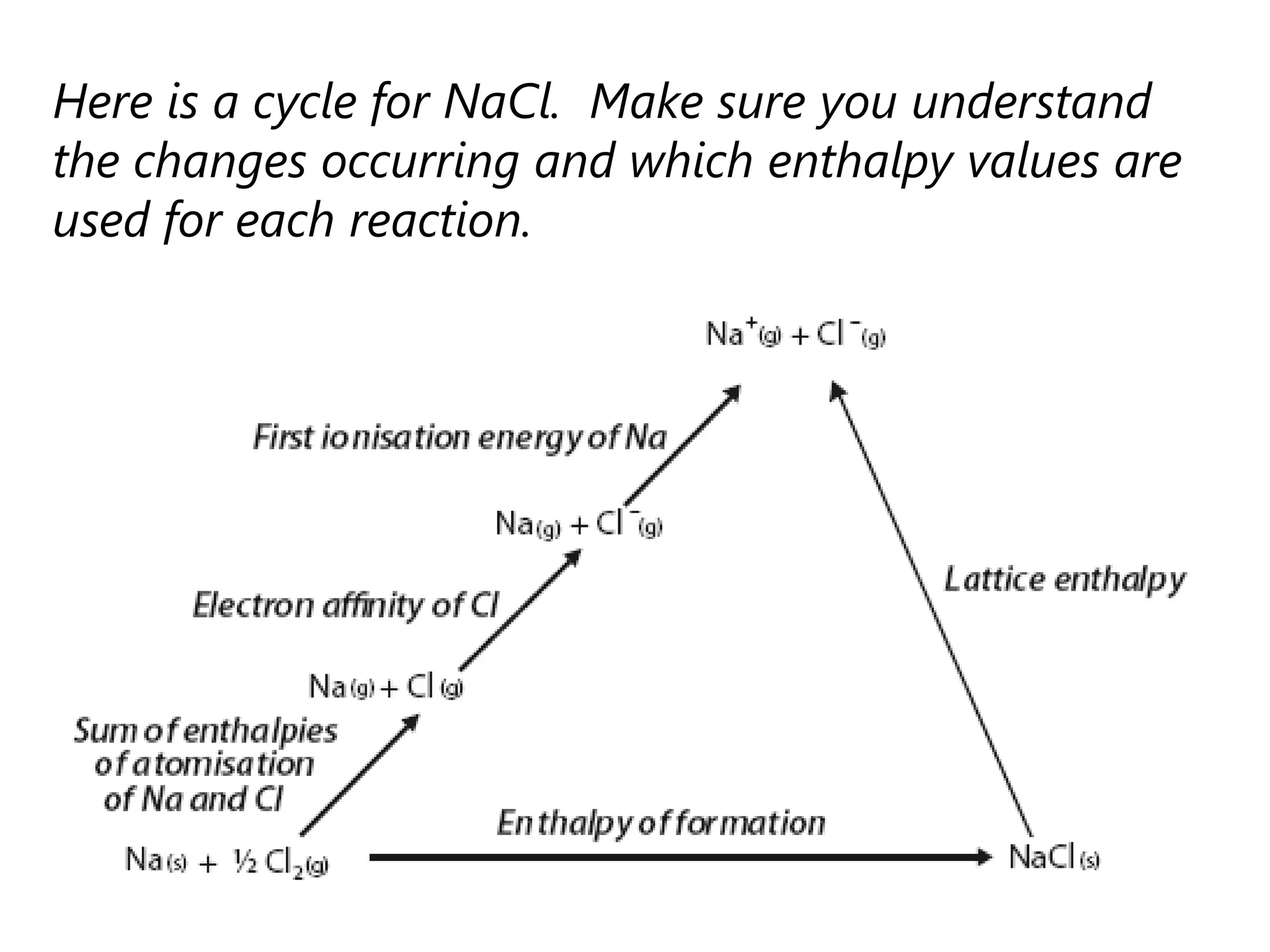
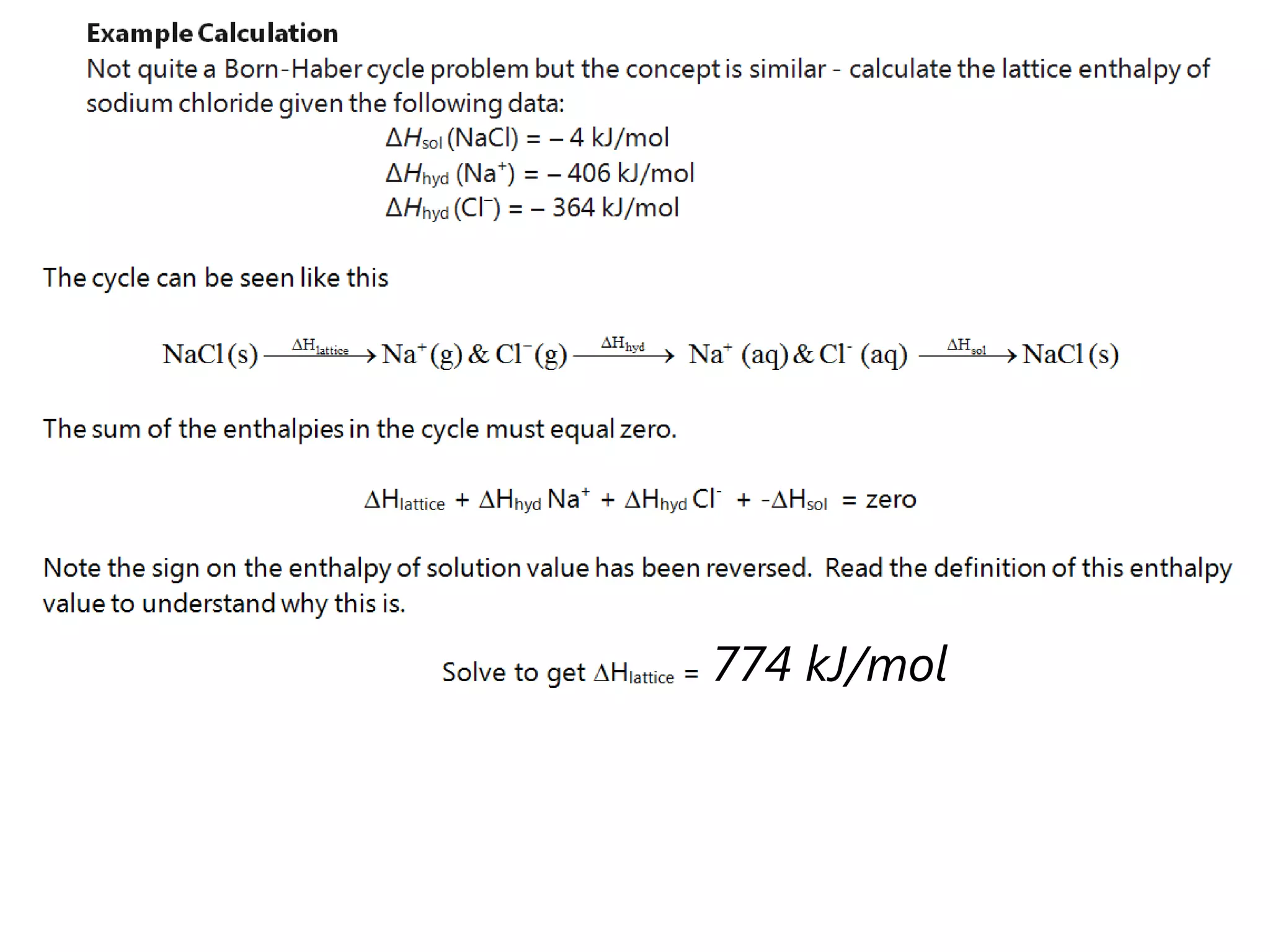
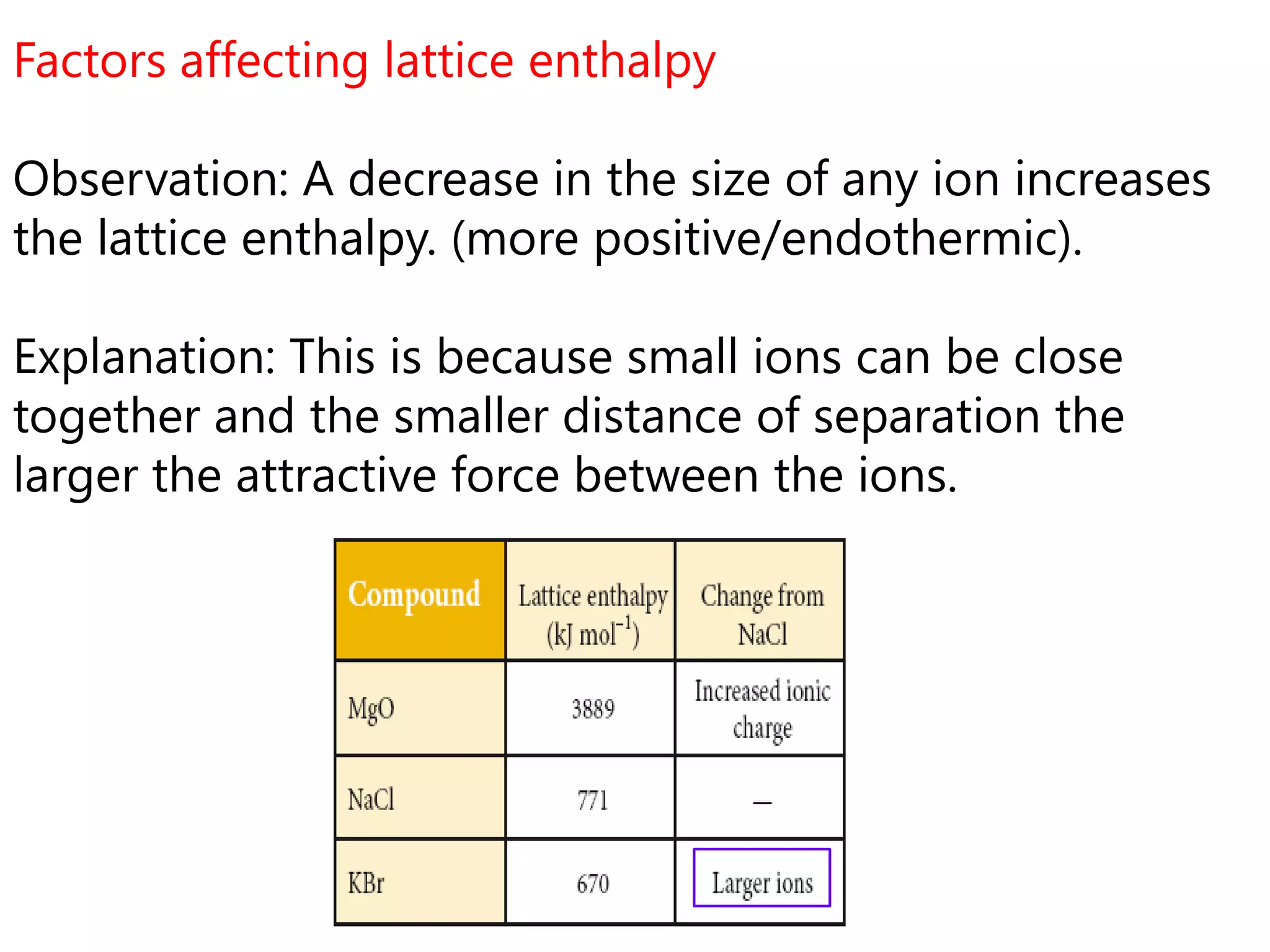
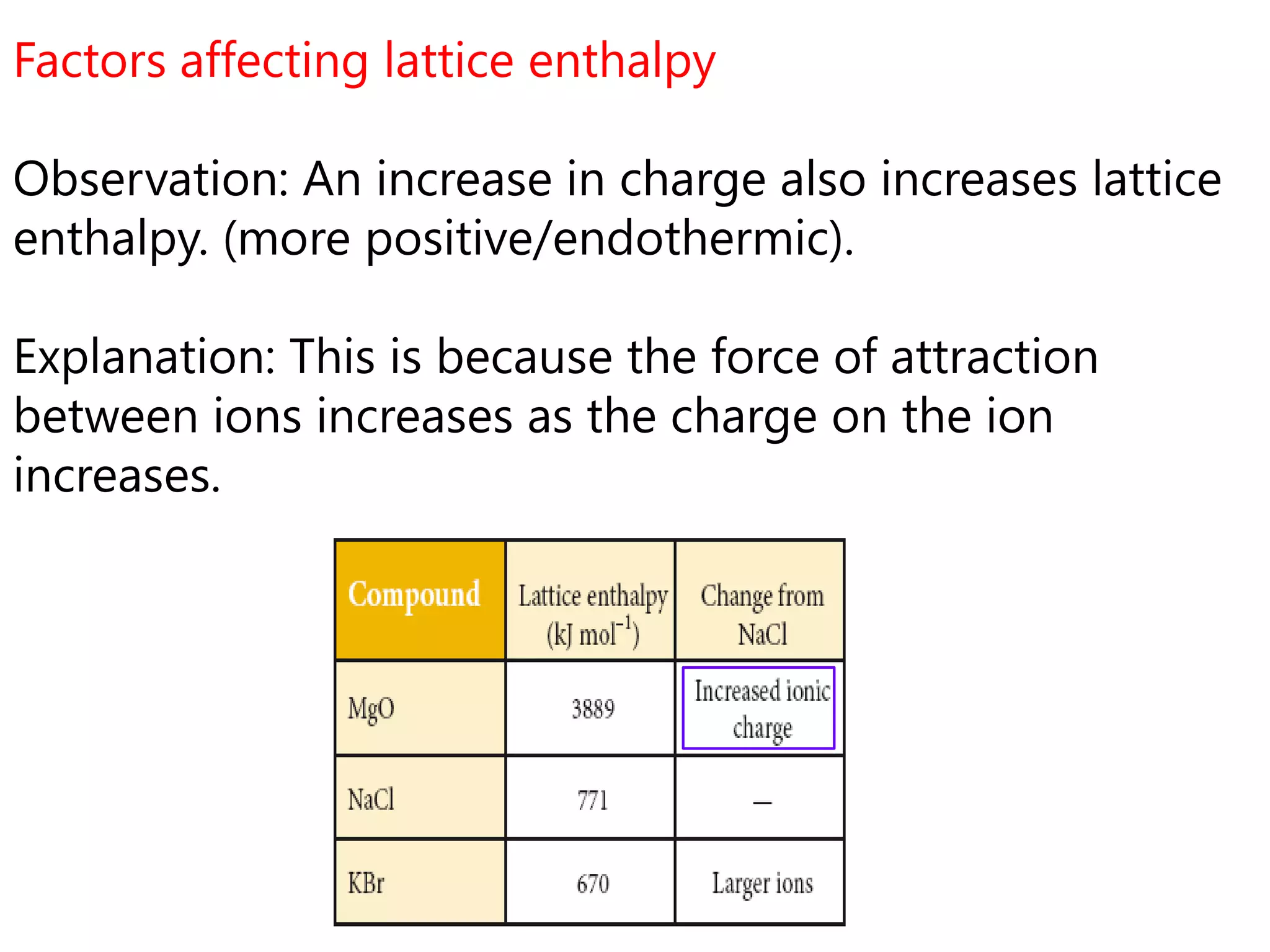
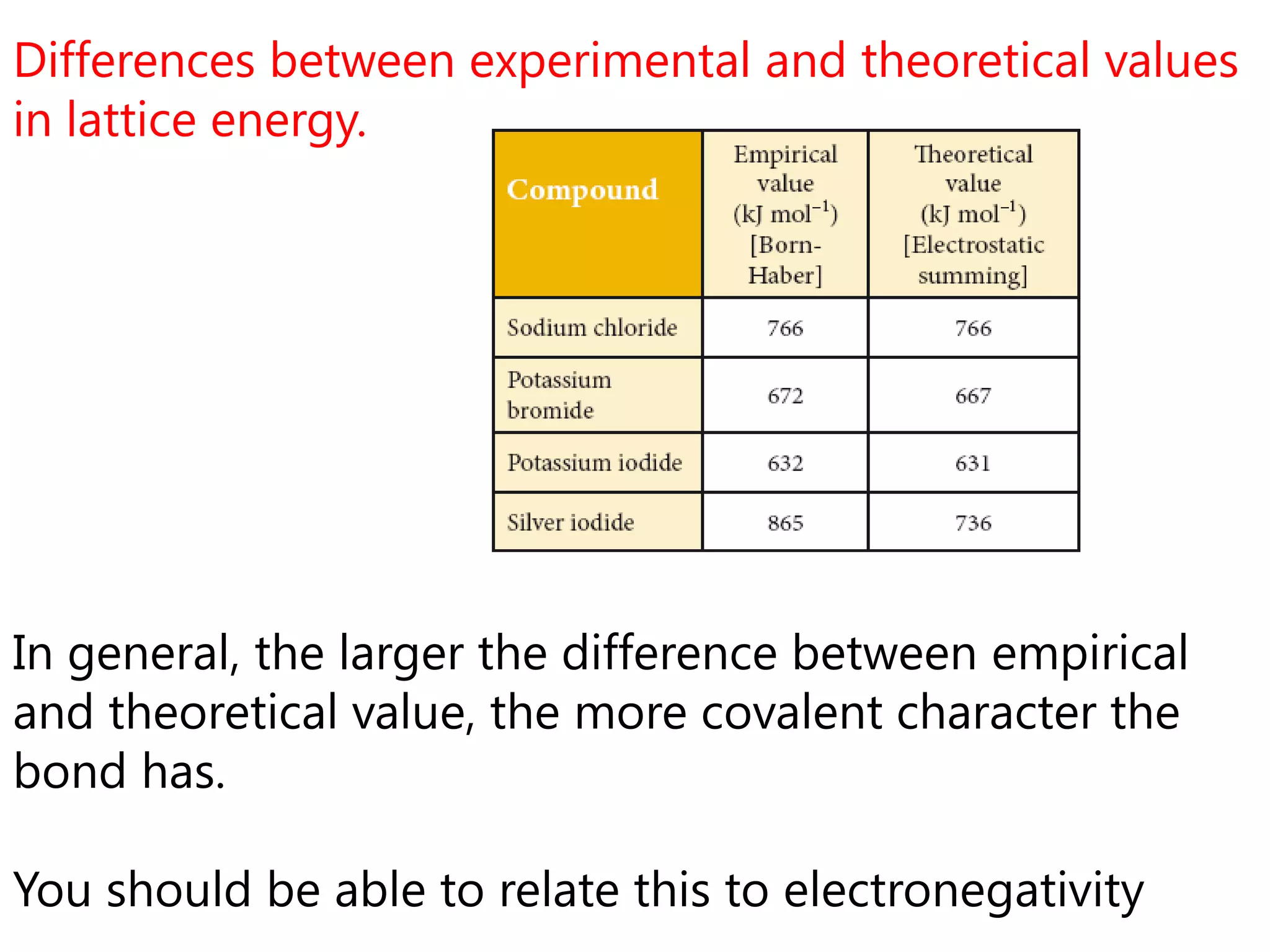
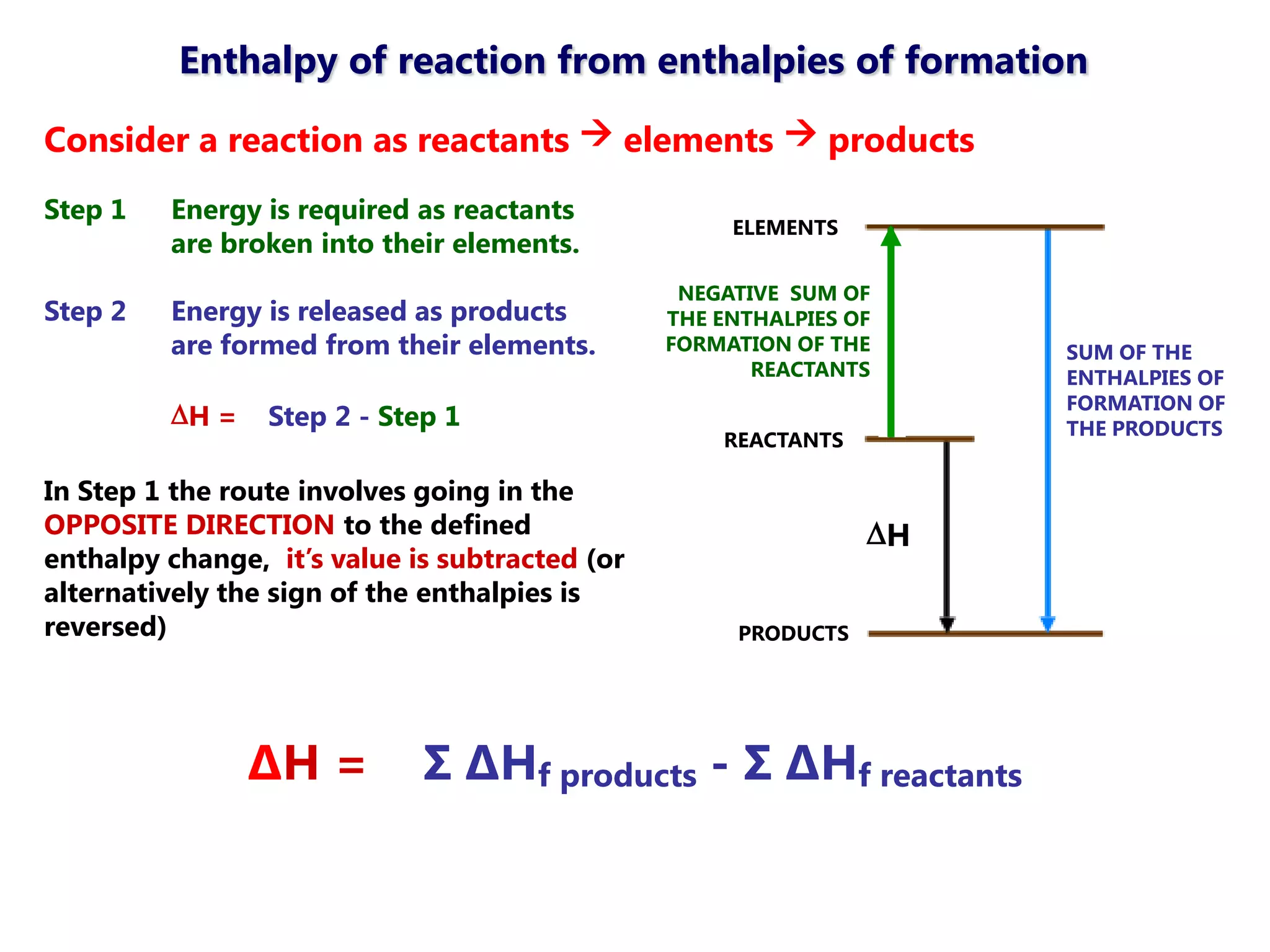
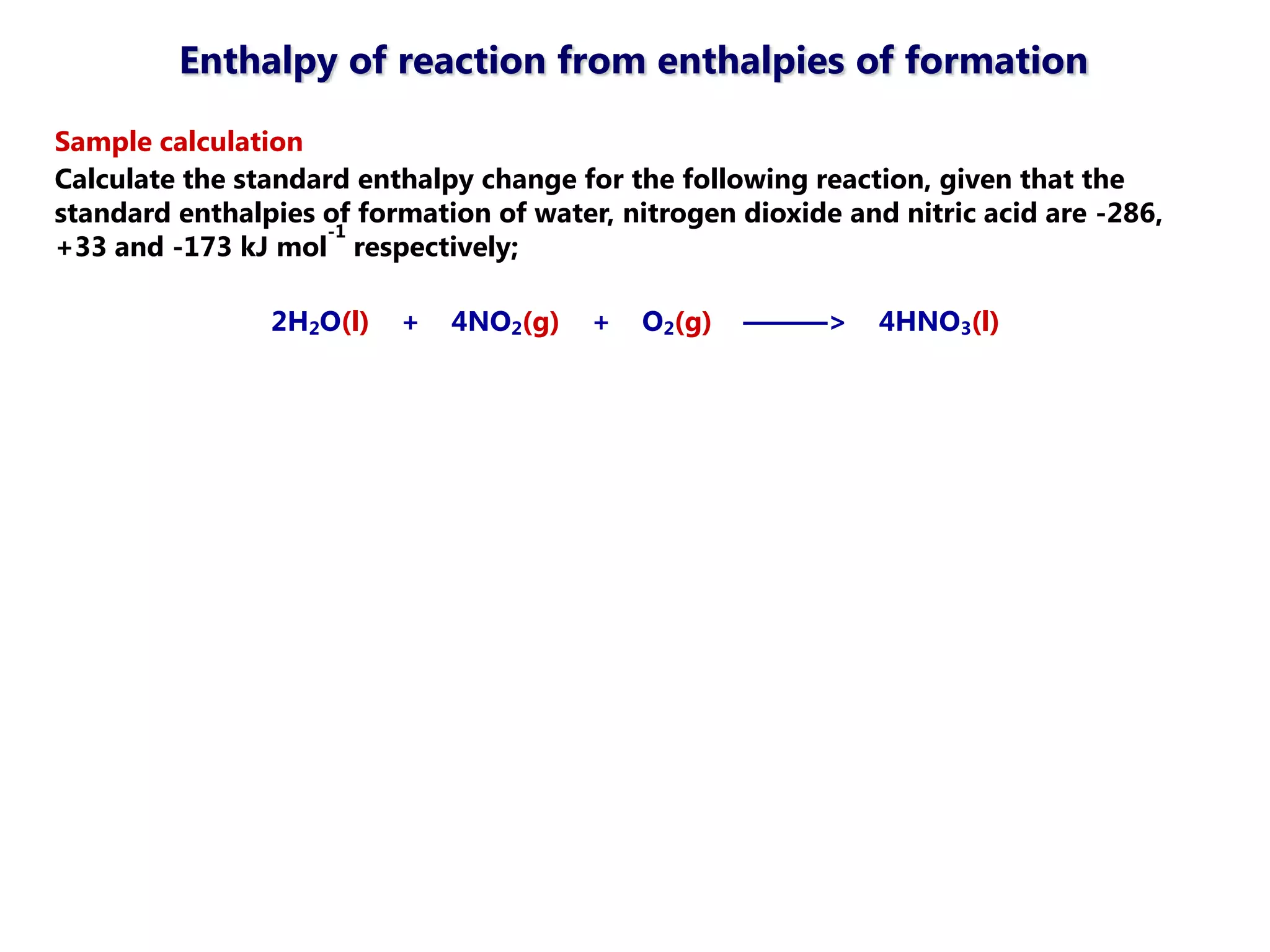
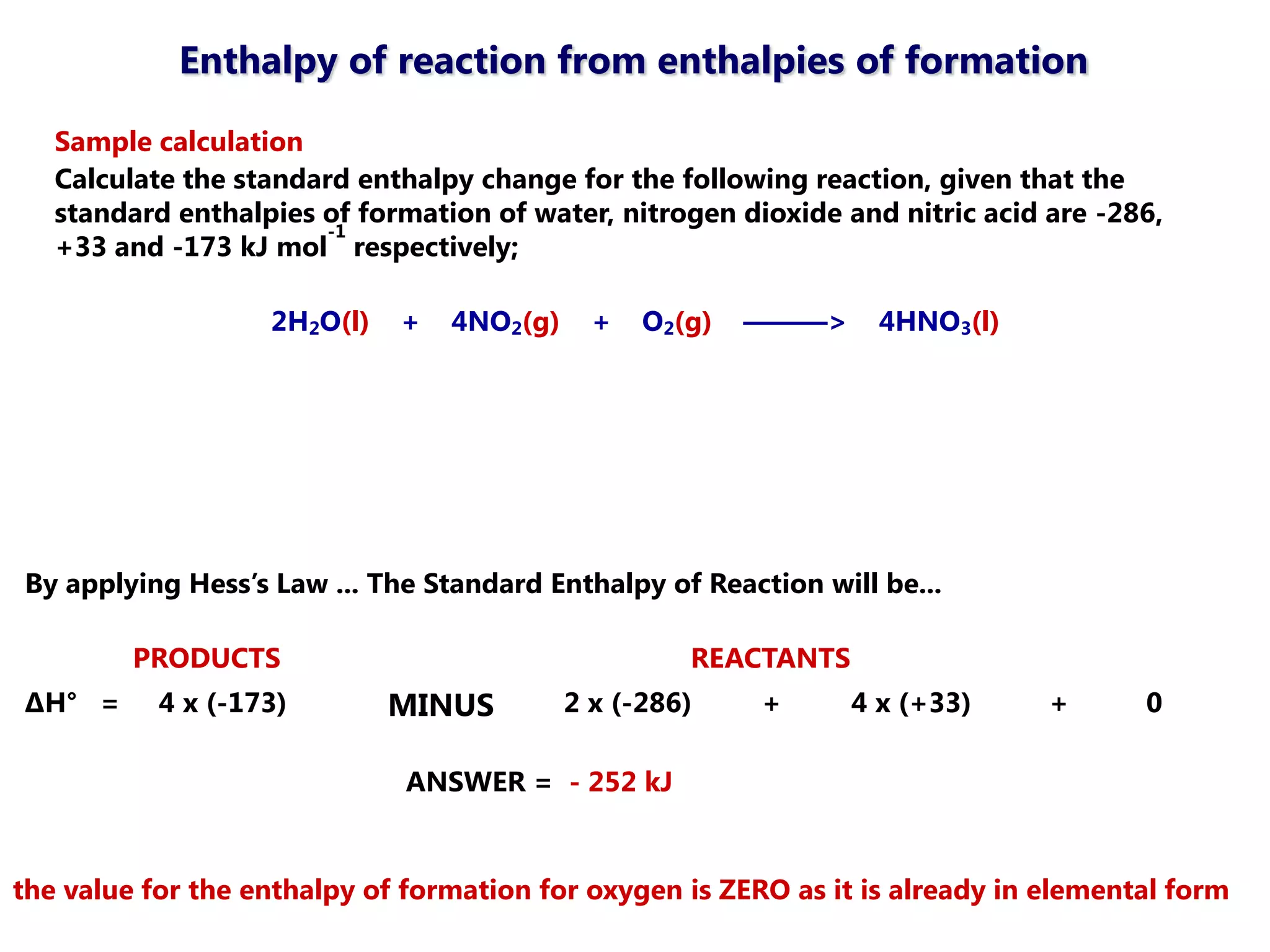
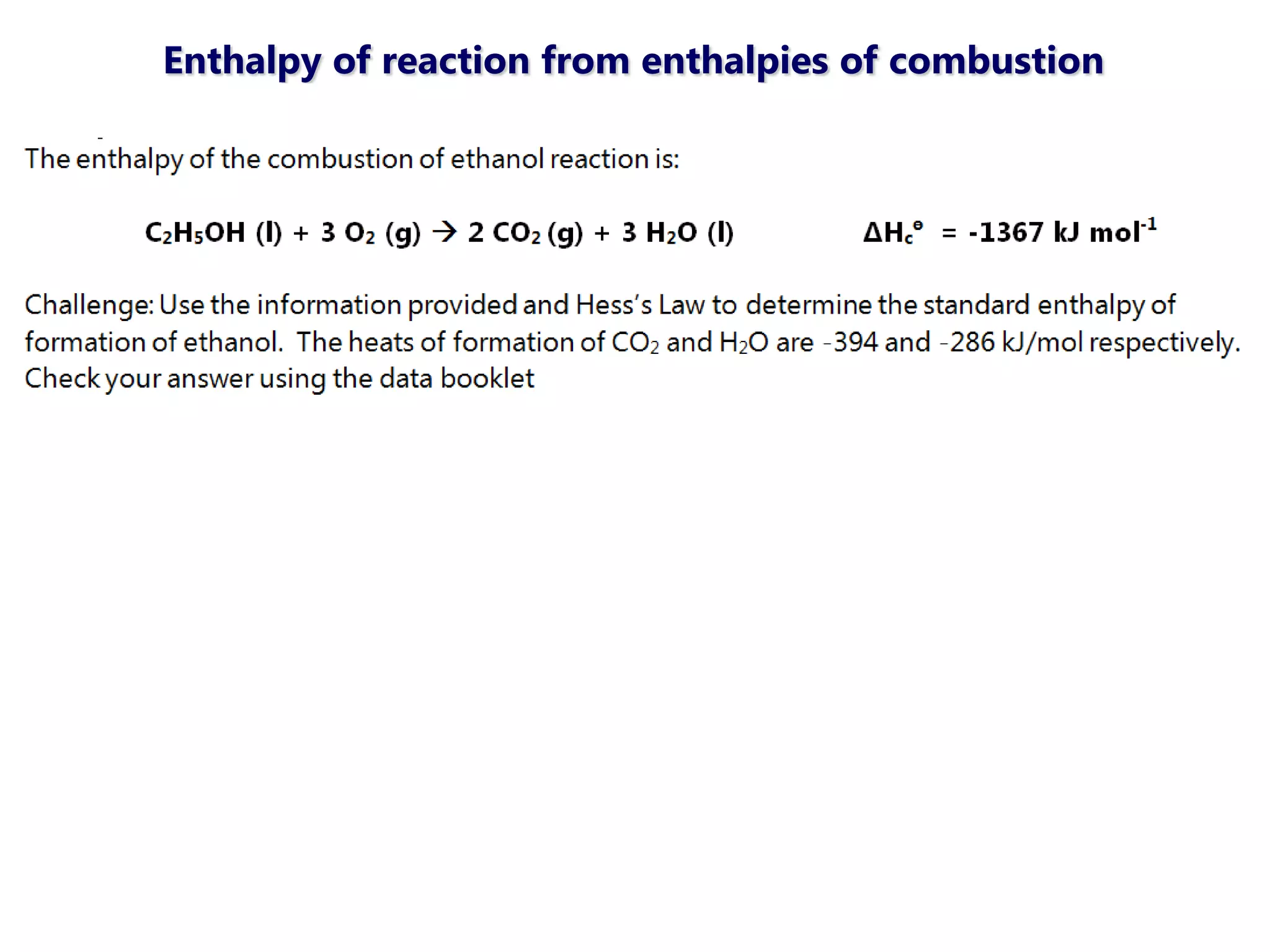
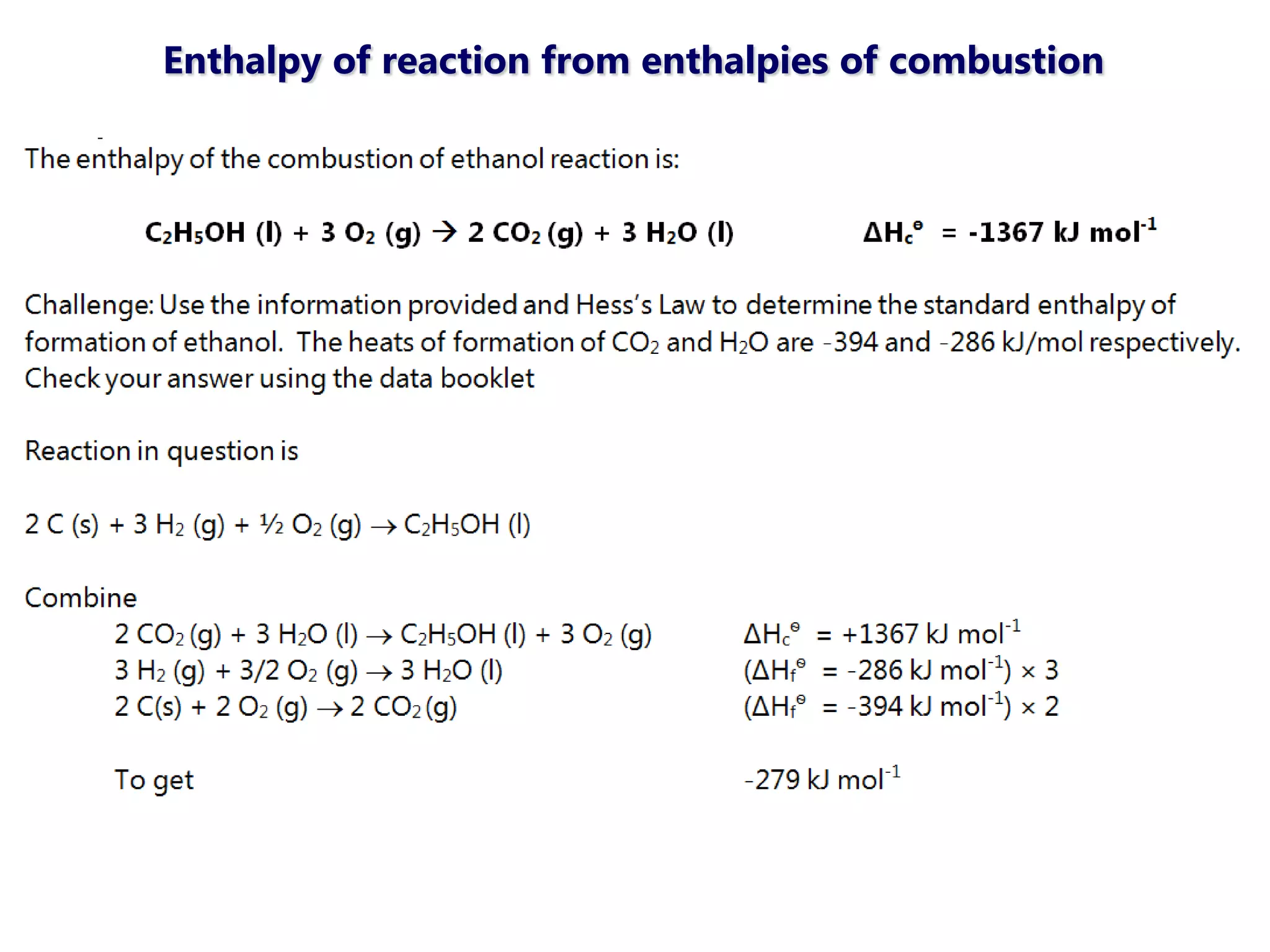
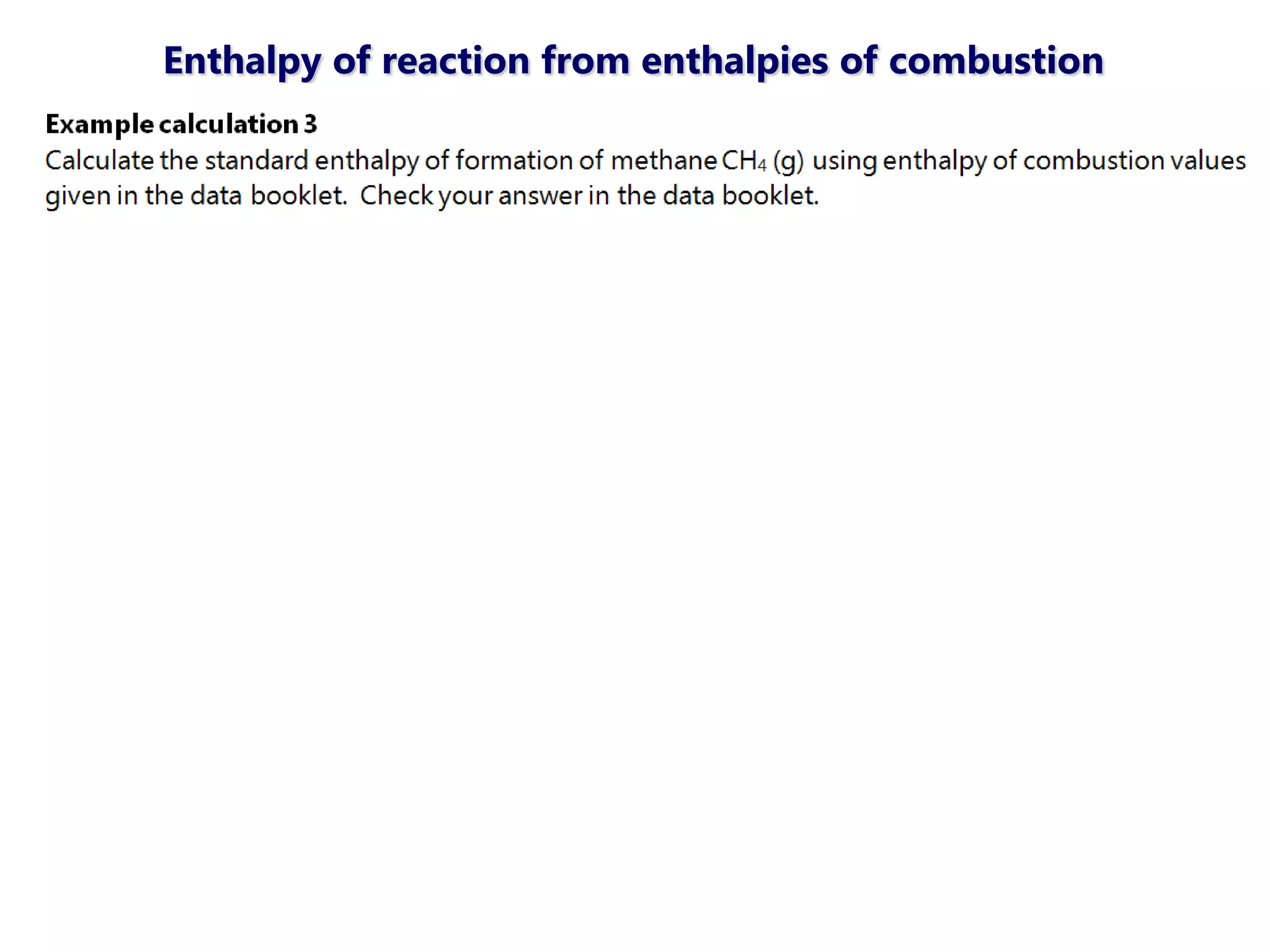
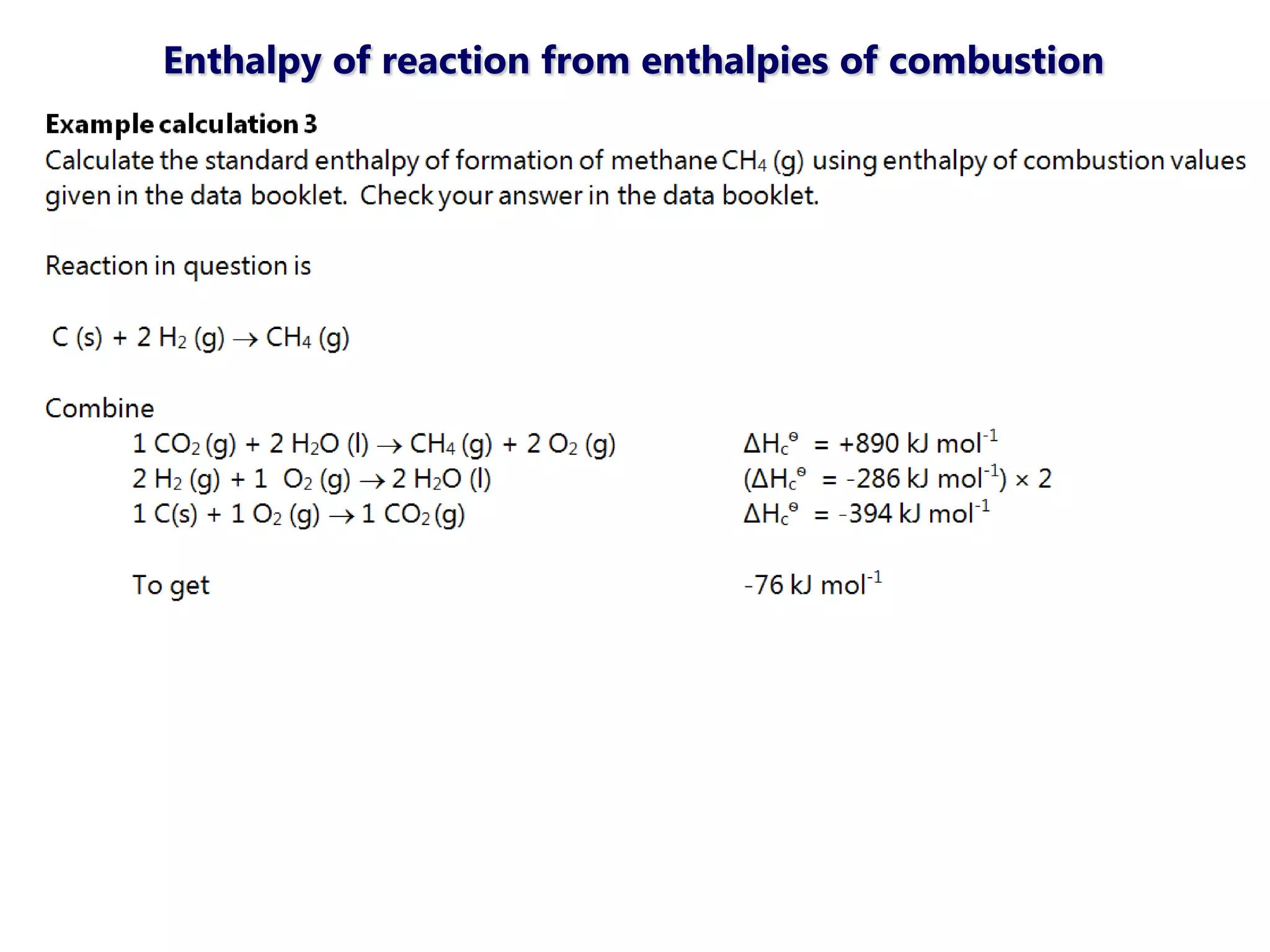
This document discusses energetics topics including standard enthalpies, Born-Haber cycles, and lattice energies. It provides an example of using enthalpies of formation to calculate the standard enthalpy change of a reaction. It also explains the Born-Haber cycle which can be used to calculate lattice energies by comparing the enthalpy of forming an ionic compound to the enthalpies of forming the constituent ions from their elements. Factors that affect lattice energy such as ion size and charge are also discussed.


![Background Information (from Wikipedia)
“The Born–Haber cycle is an approach to analyzing
reaction energies. It was named after and developed by the
two German scientists Max Born and Fritz Haber.
The Born–Haber cycle involves the formation of an ionic
compound from the reaction of a metal (often a Group I or Group
II element) with a non-metal. Born–Haber cycles are used primarily
as a means of calculating lattice energies (or more precisely
[1]
enthalpies ) which cannot otherwise be measured directly.
The lattice enthalpy is the enthalpy change involved in formation
of the ionic compound from gaseous ions. Some chemists define it
as the energy to break the ionic compound into gaseous ions. The
former definition is invariably exothermic and the latter is
endothermic.”](https://image.slidesharecdn.com/2012151and152-120401233617-phpapp01/75/2012-15-1-and-15-2-11-2048.jpg)