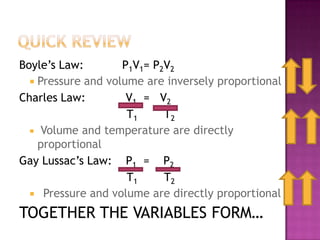
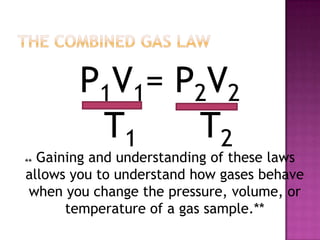
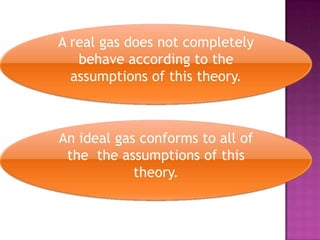
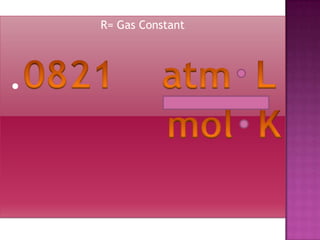
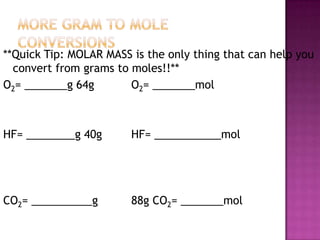
The document discusses the ideal gas law and its relationship between the pressure, volume, temperature, and amount of gas. It defines Boyle's law, Charles' law, Gay-Lussac's law, and how they combine to form the ideal gas law (PV=nRT). It provides the definitions and units for pressure, volume, temperature, moles, and the gas constant in the ideal gas law. Examples are given for converting between pressure units and using the ideal gas law to calculate moles or temperature given the other variables.