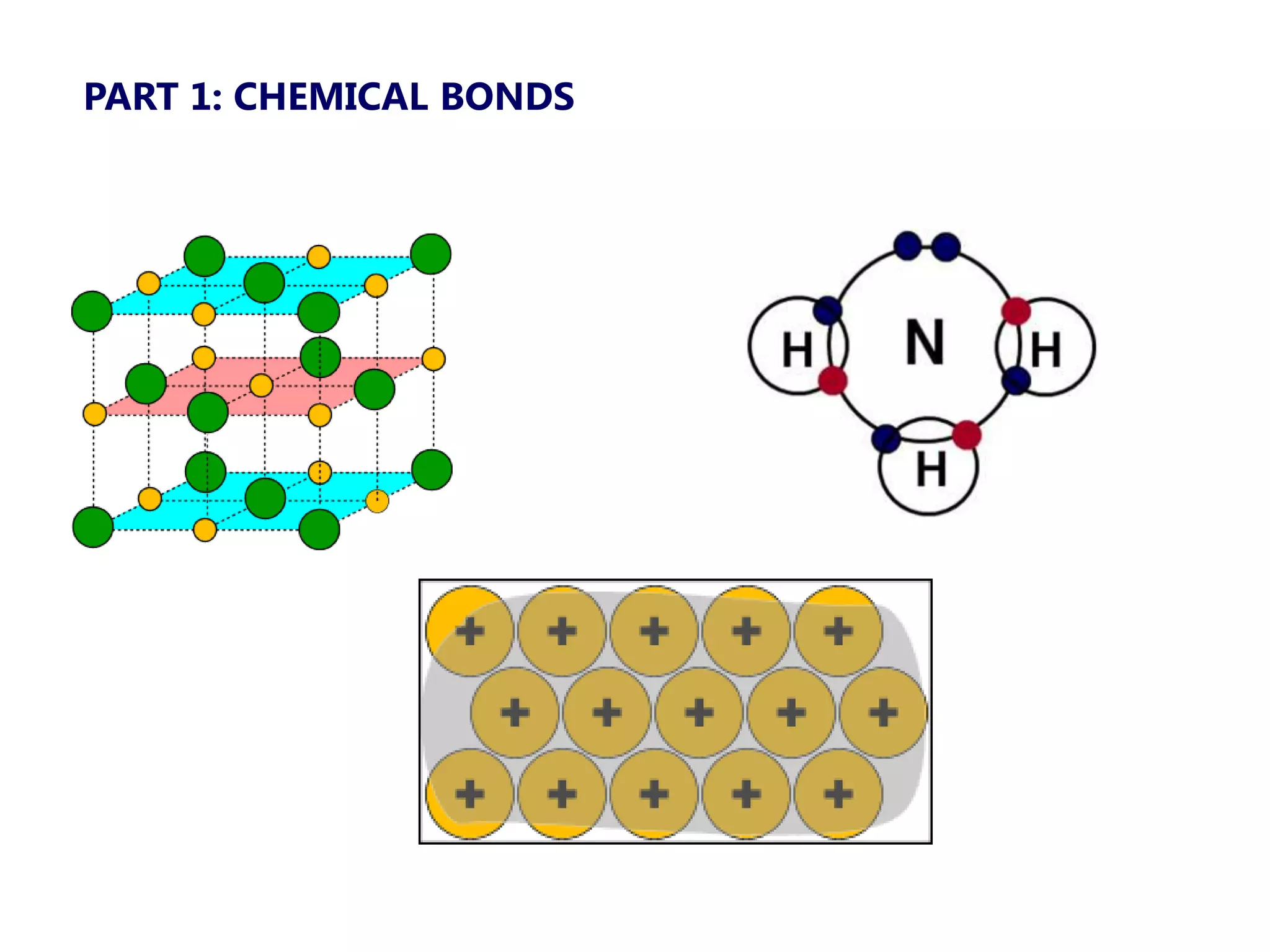
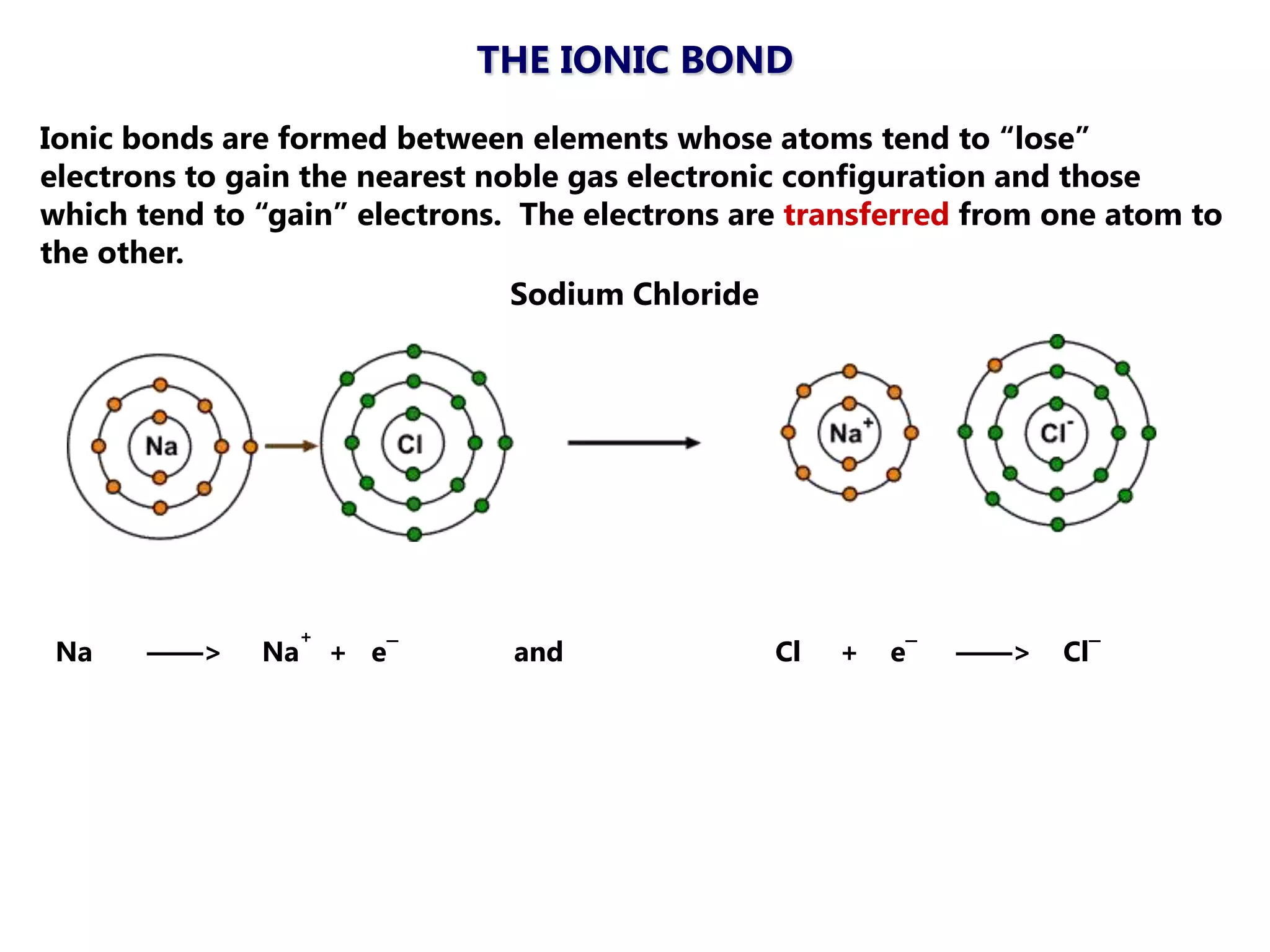
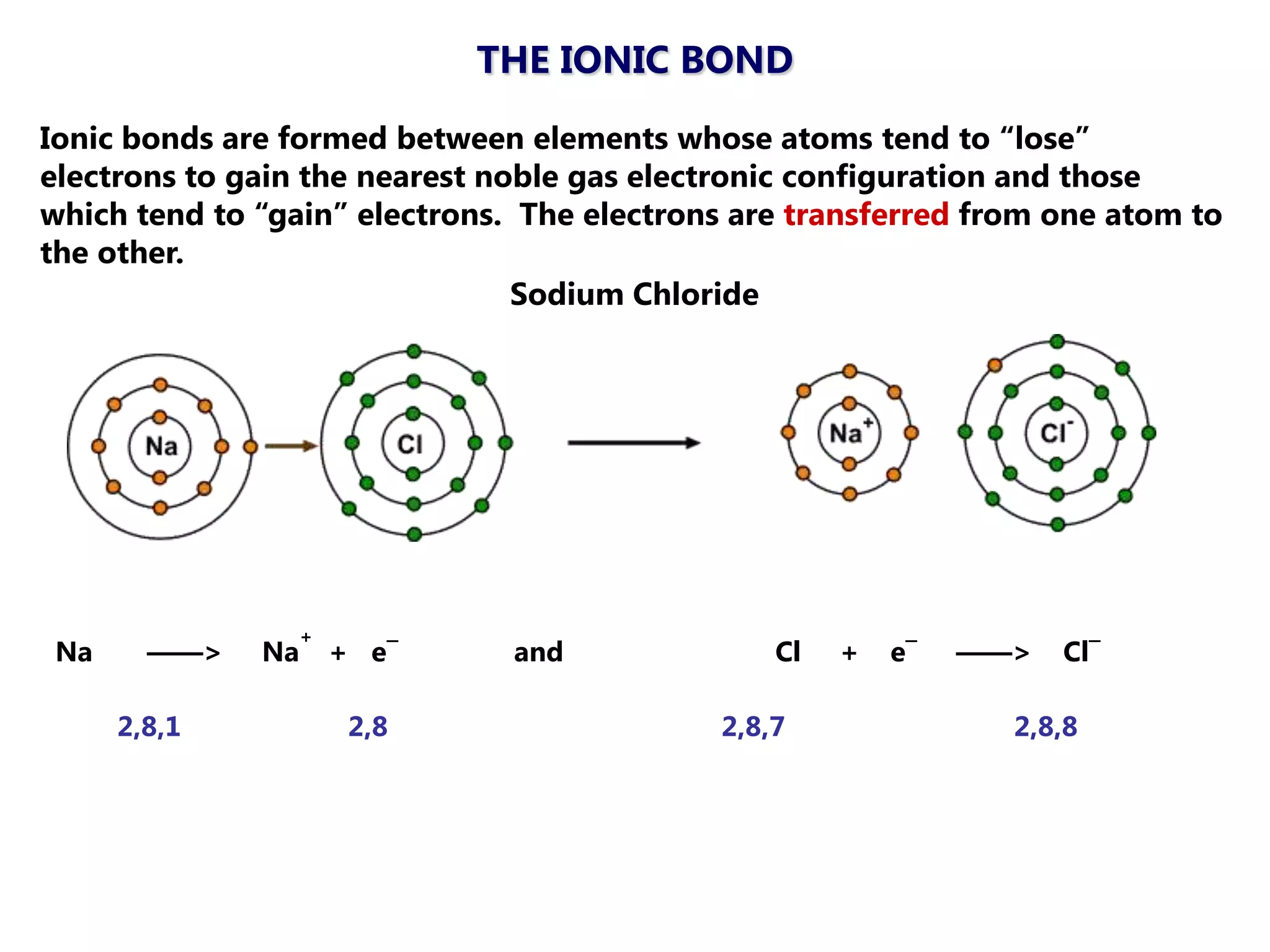
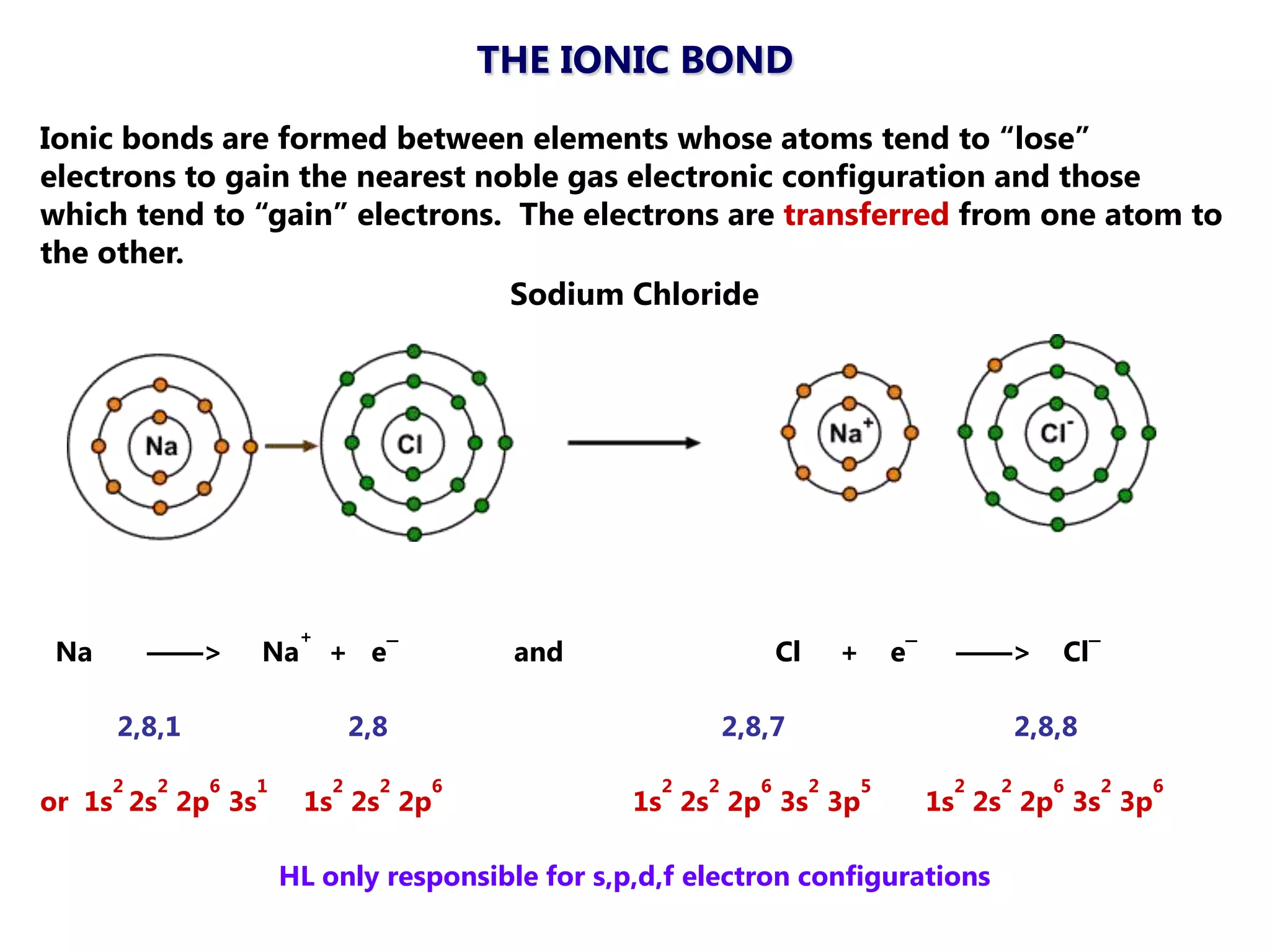
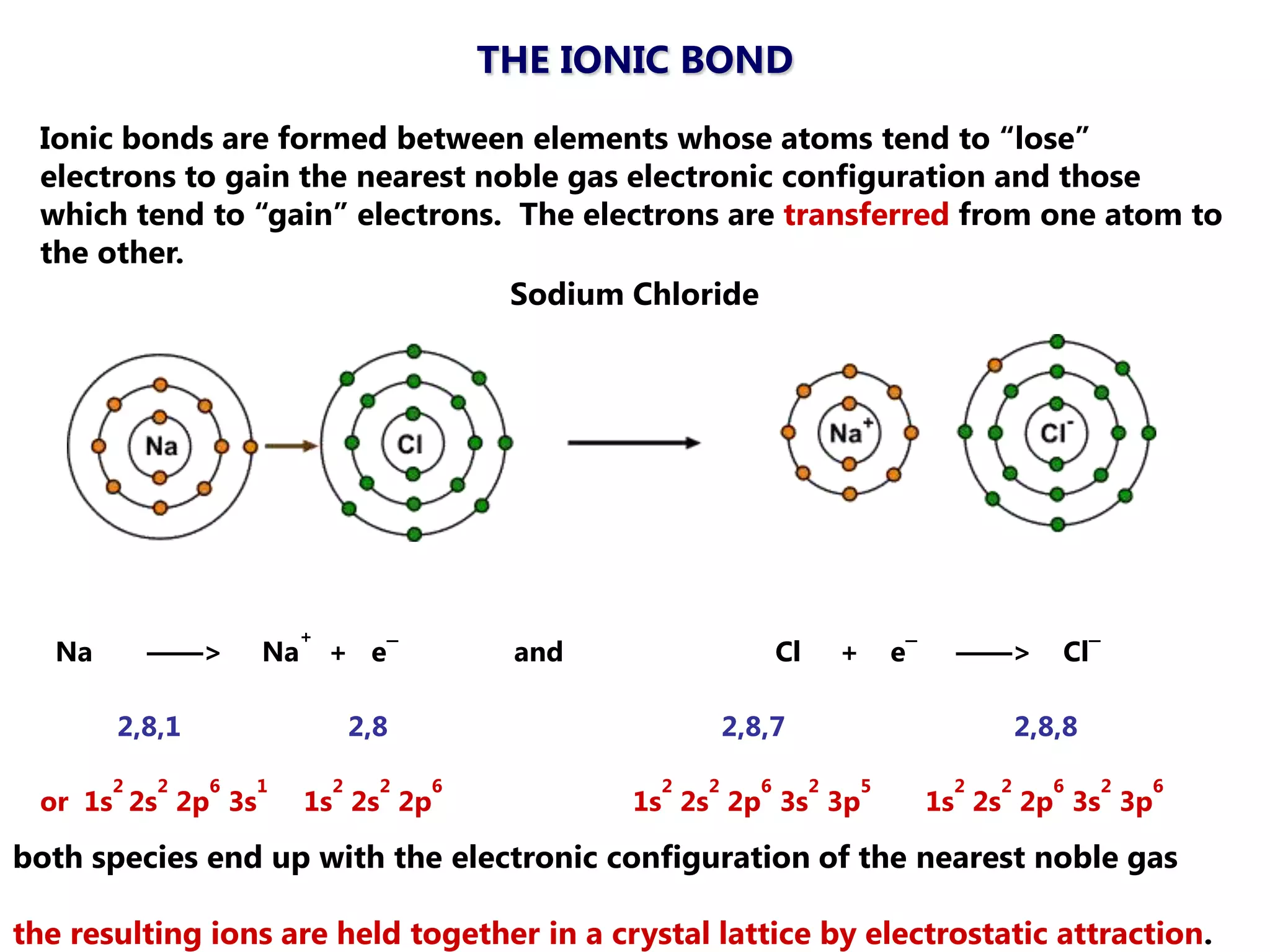
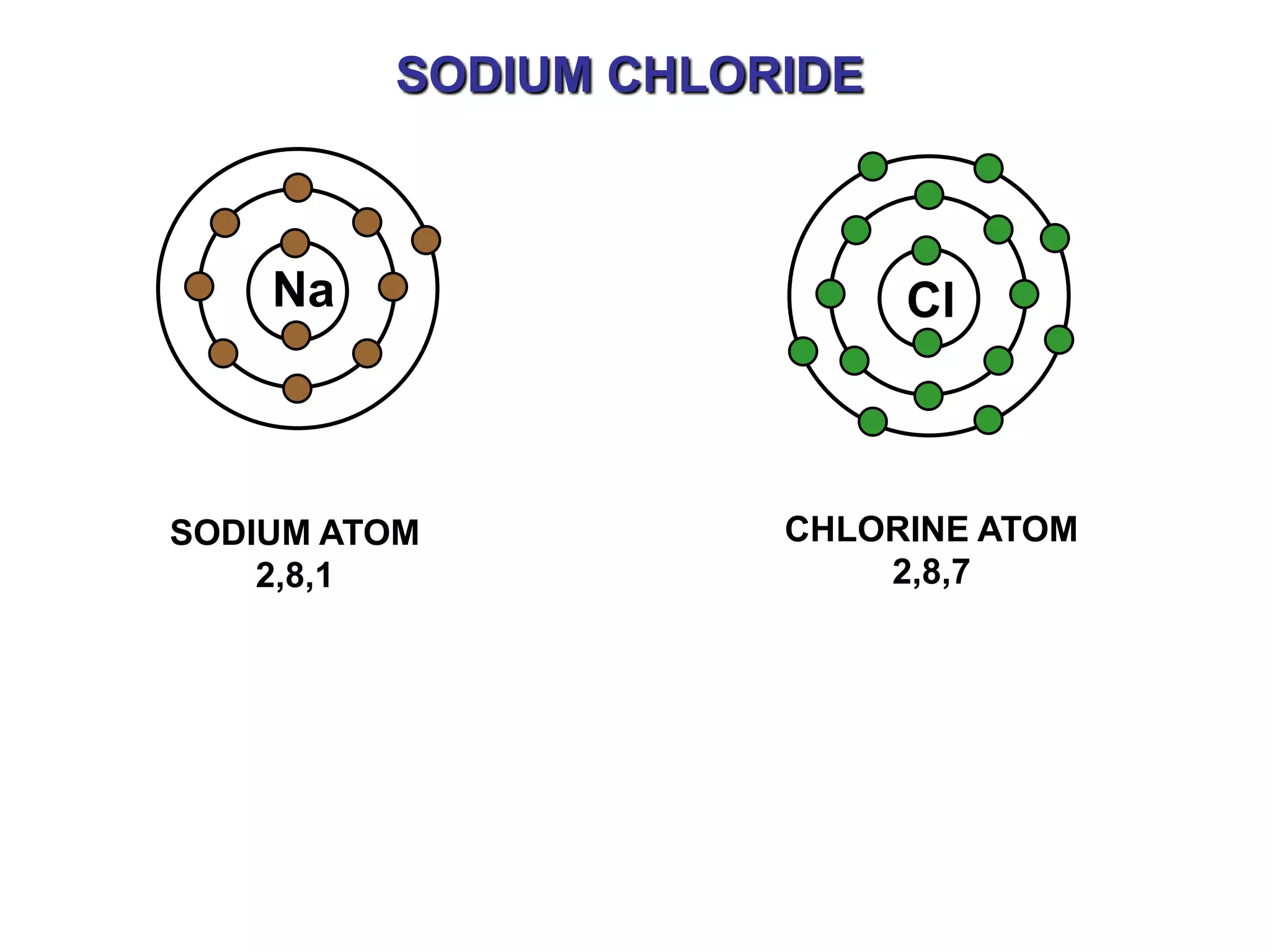
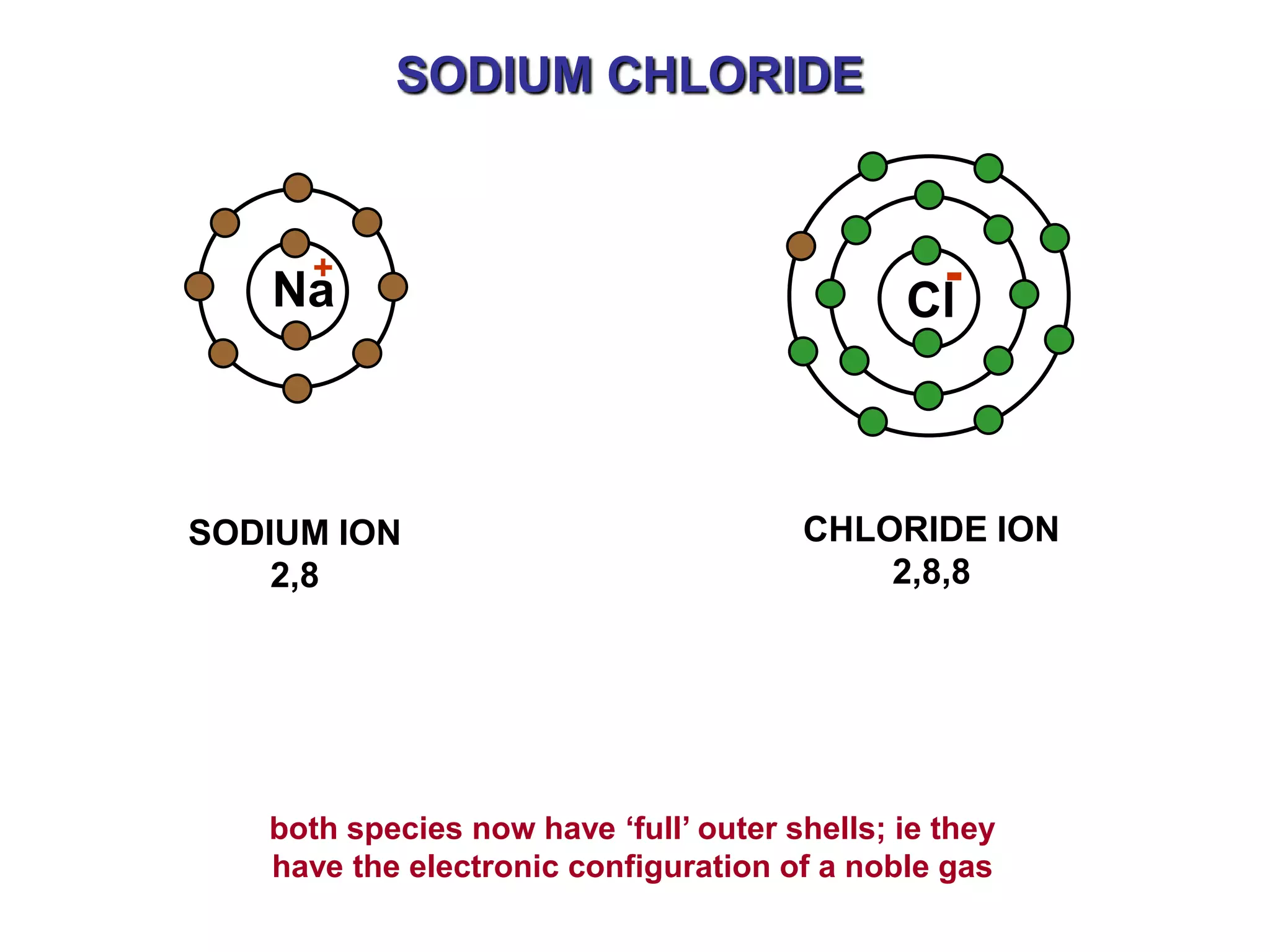
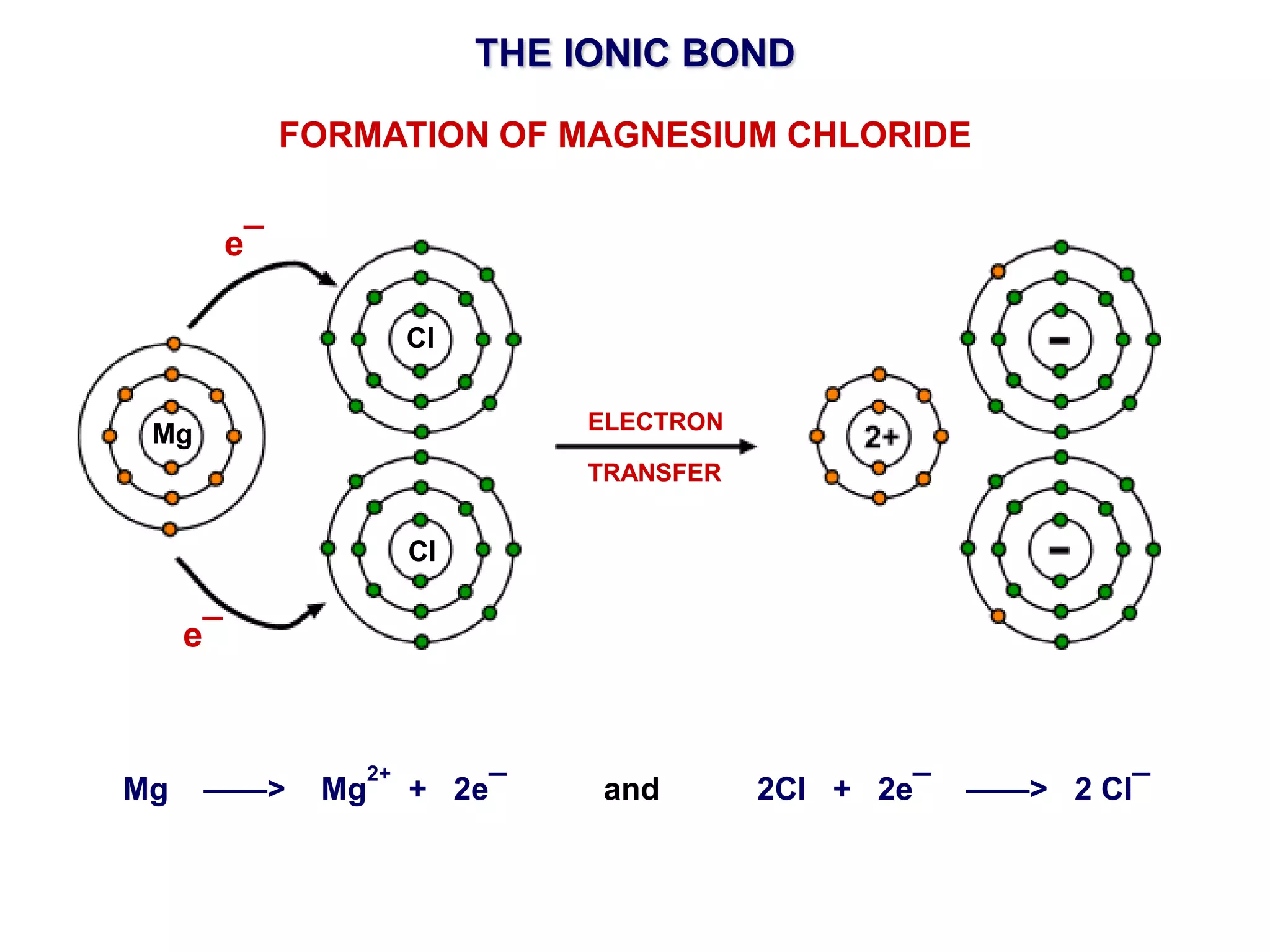
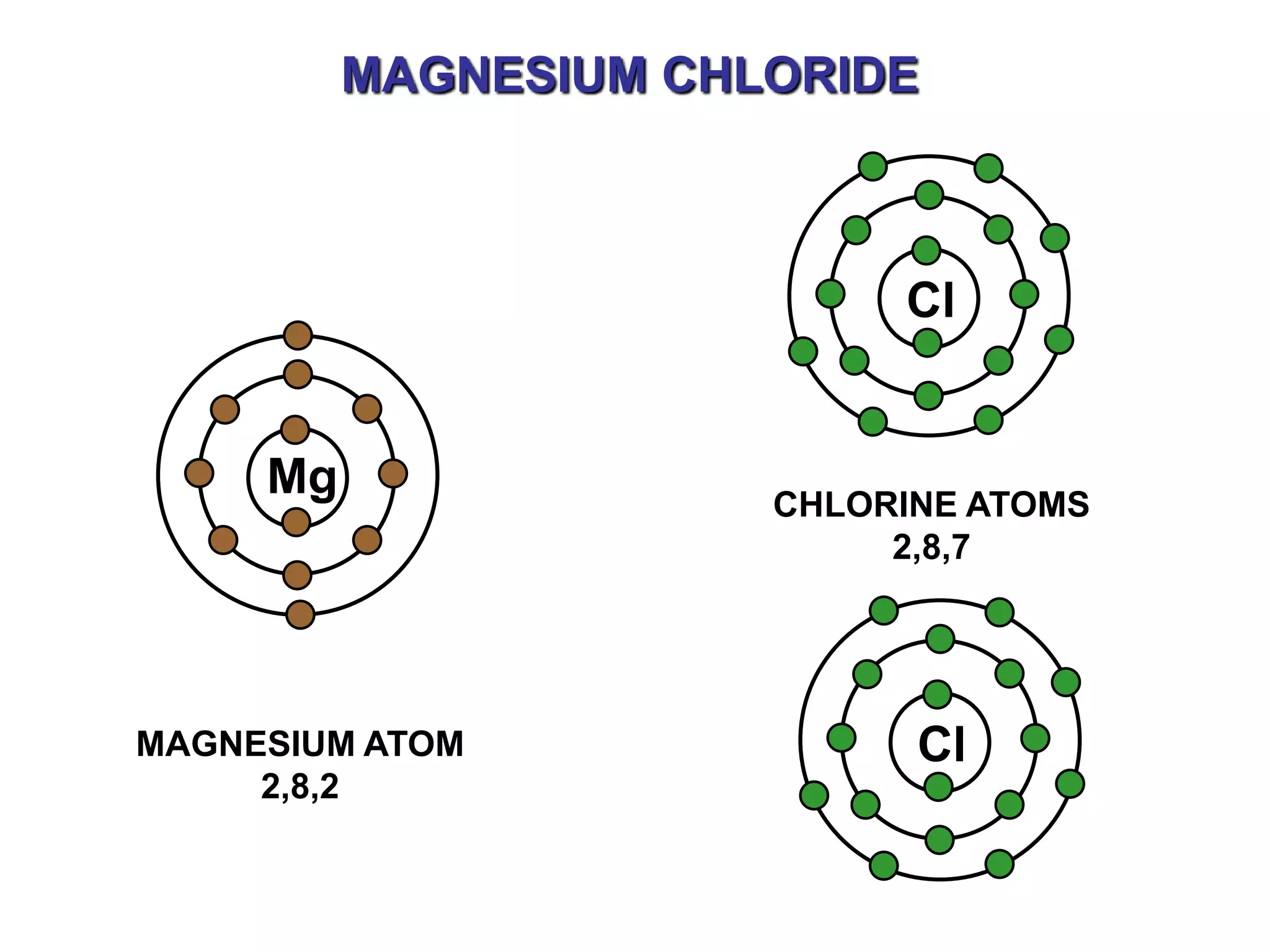
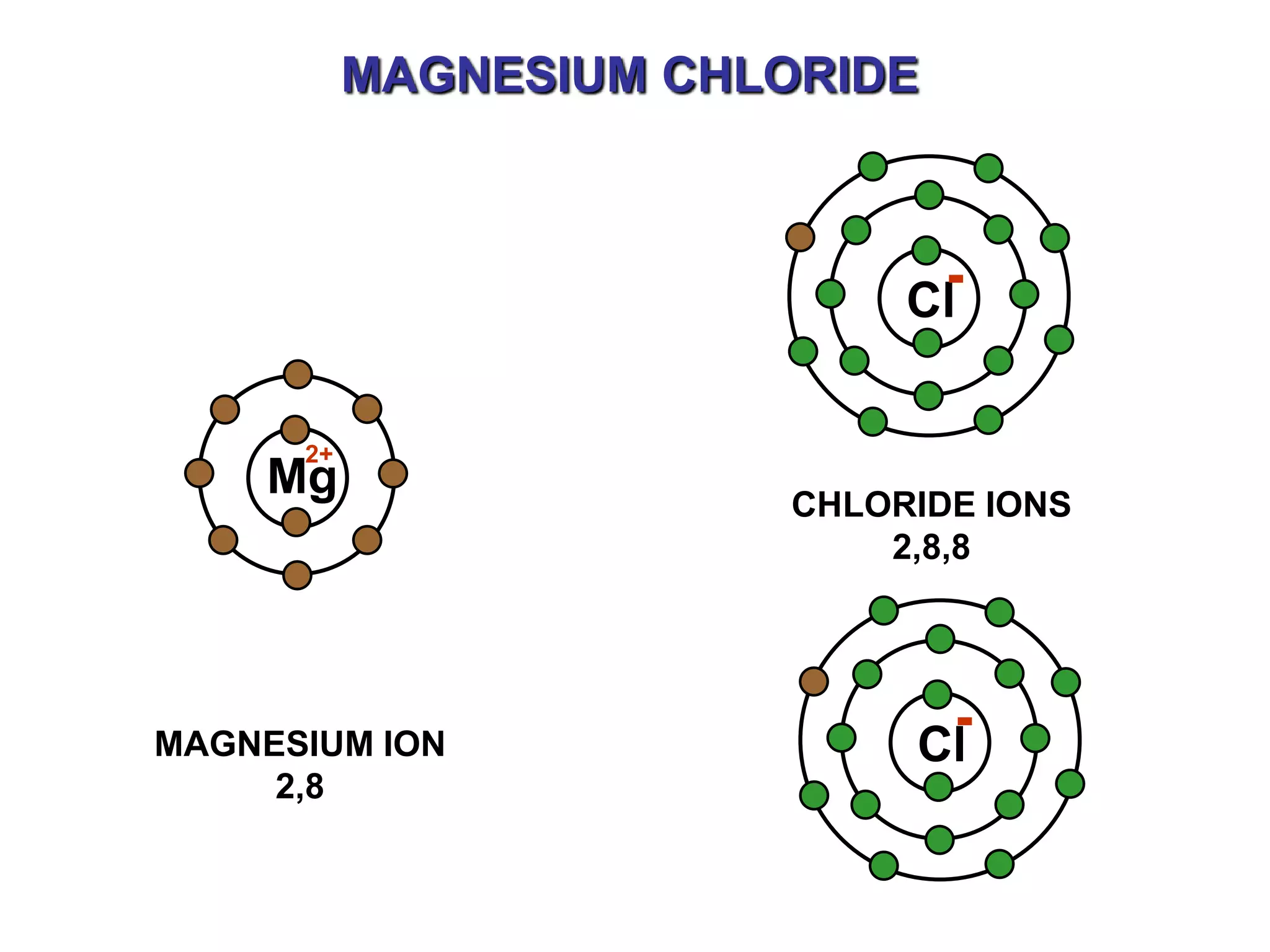
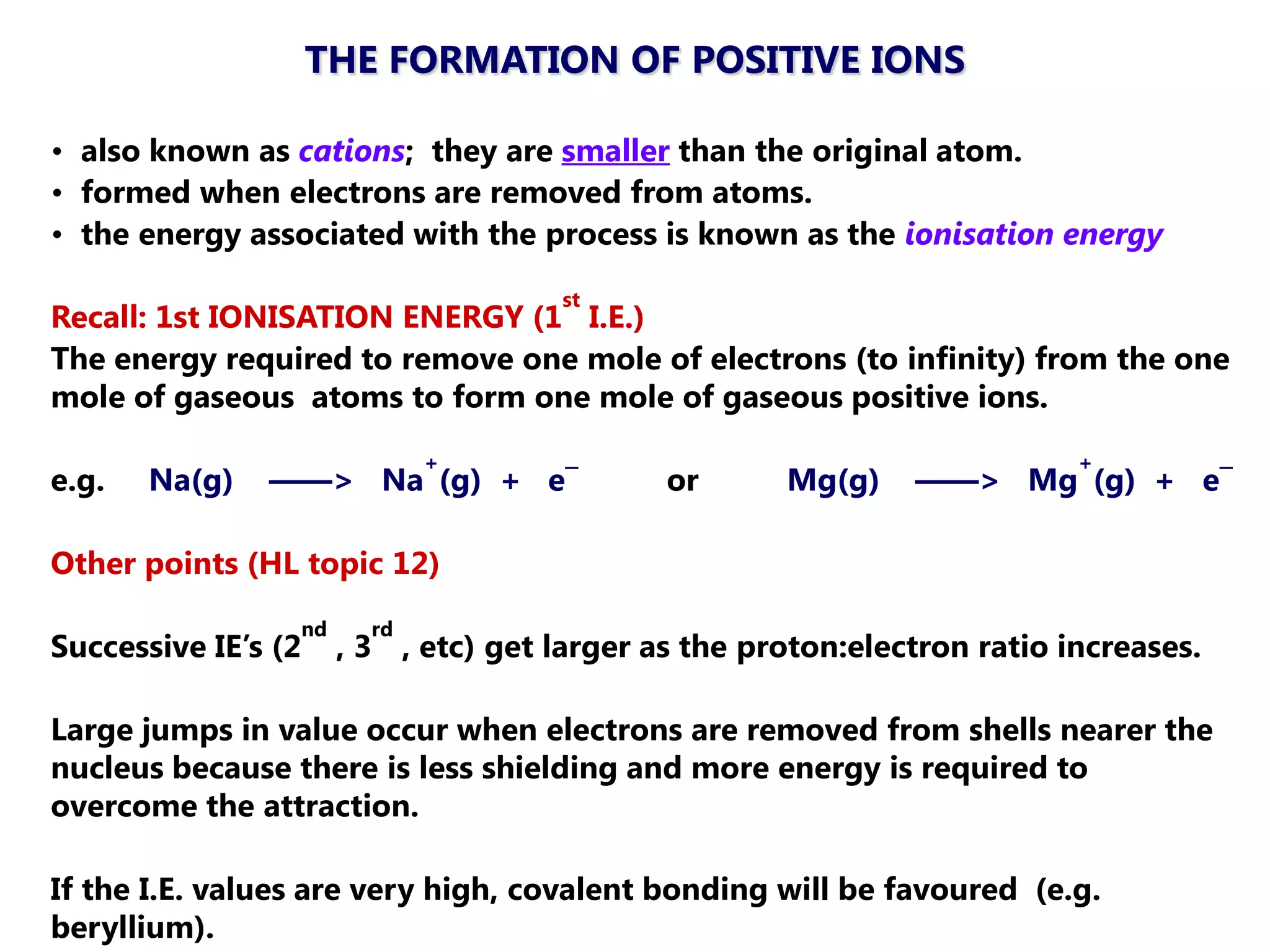
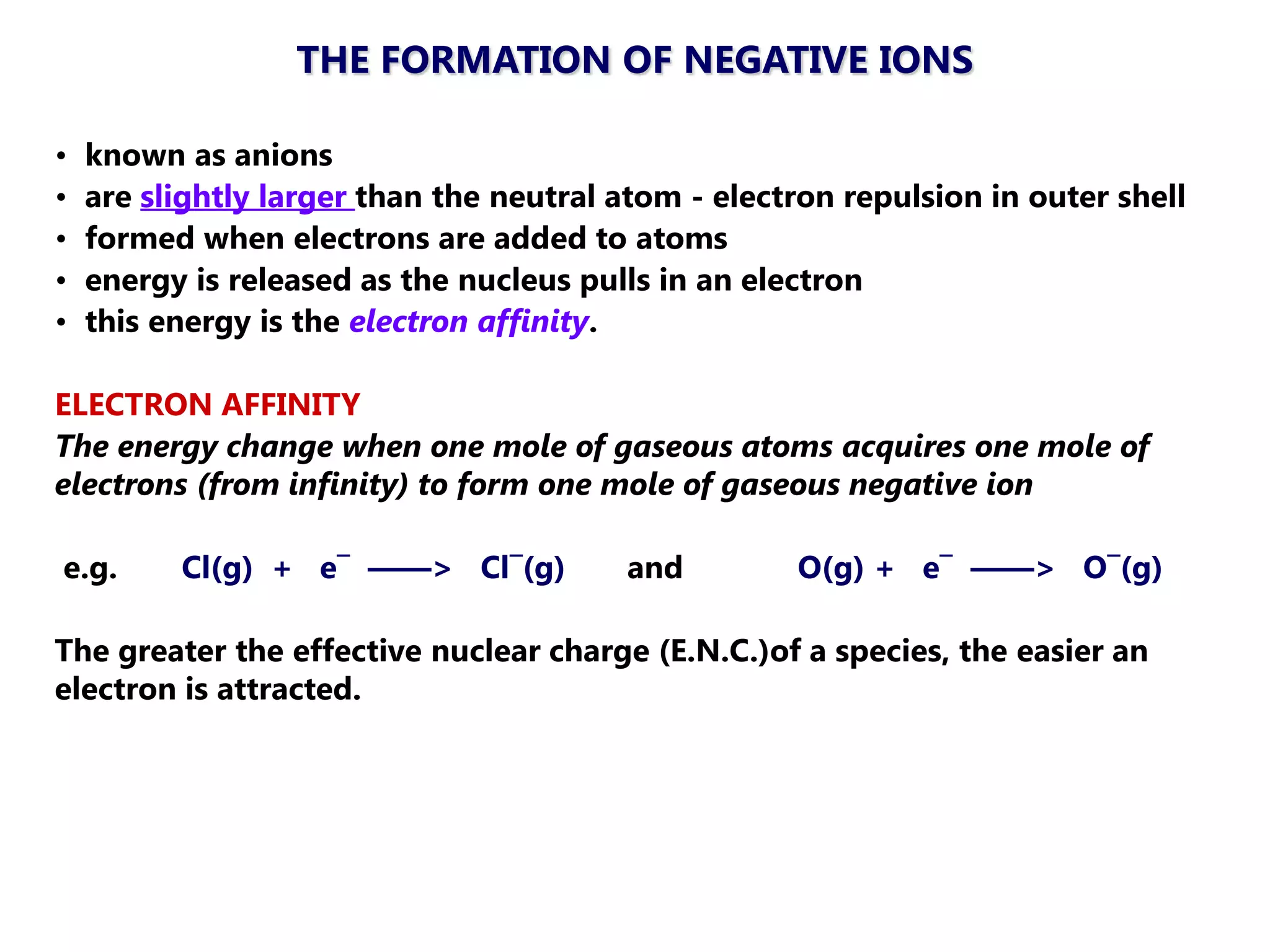
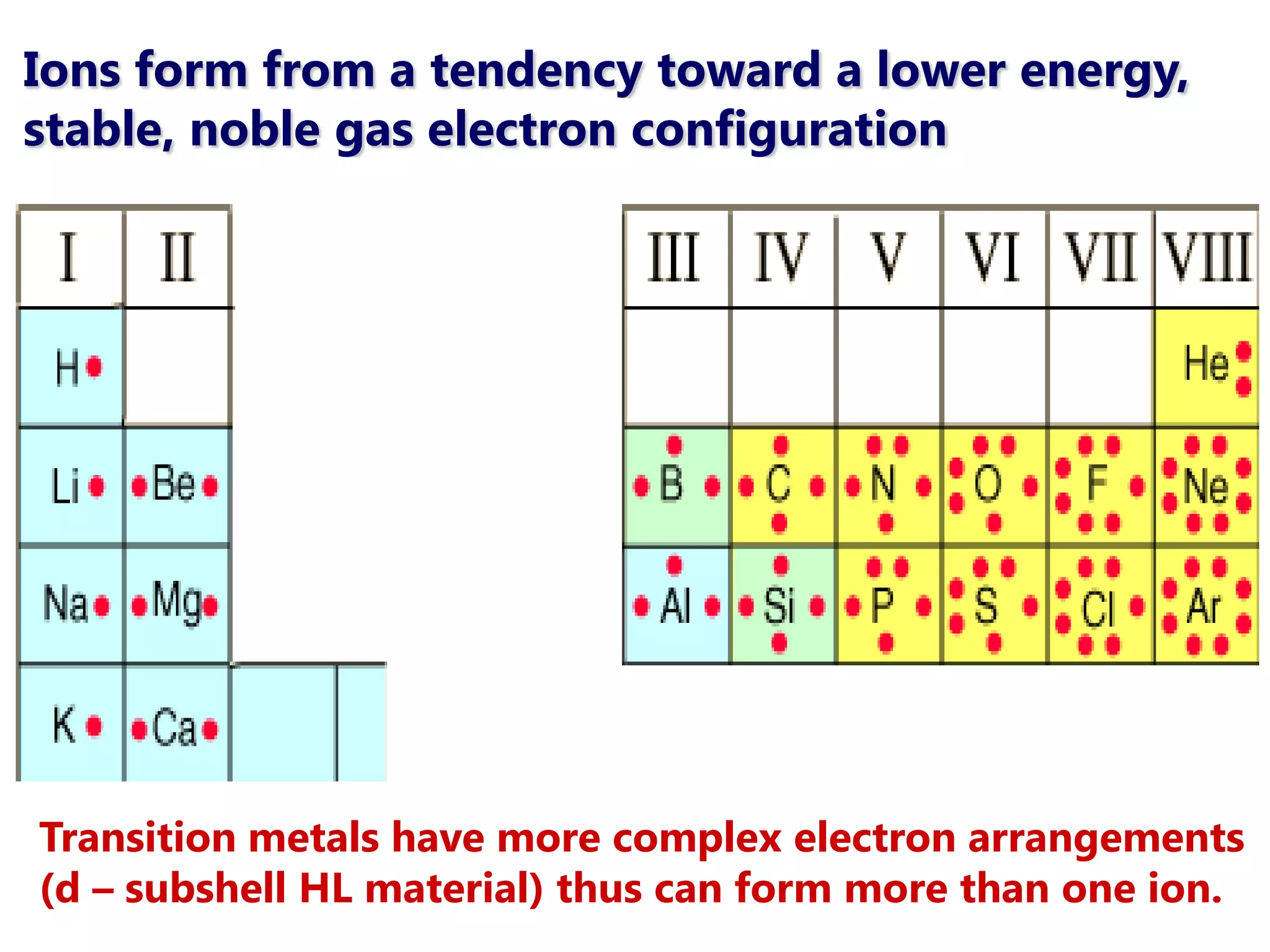
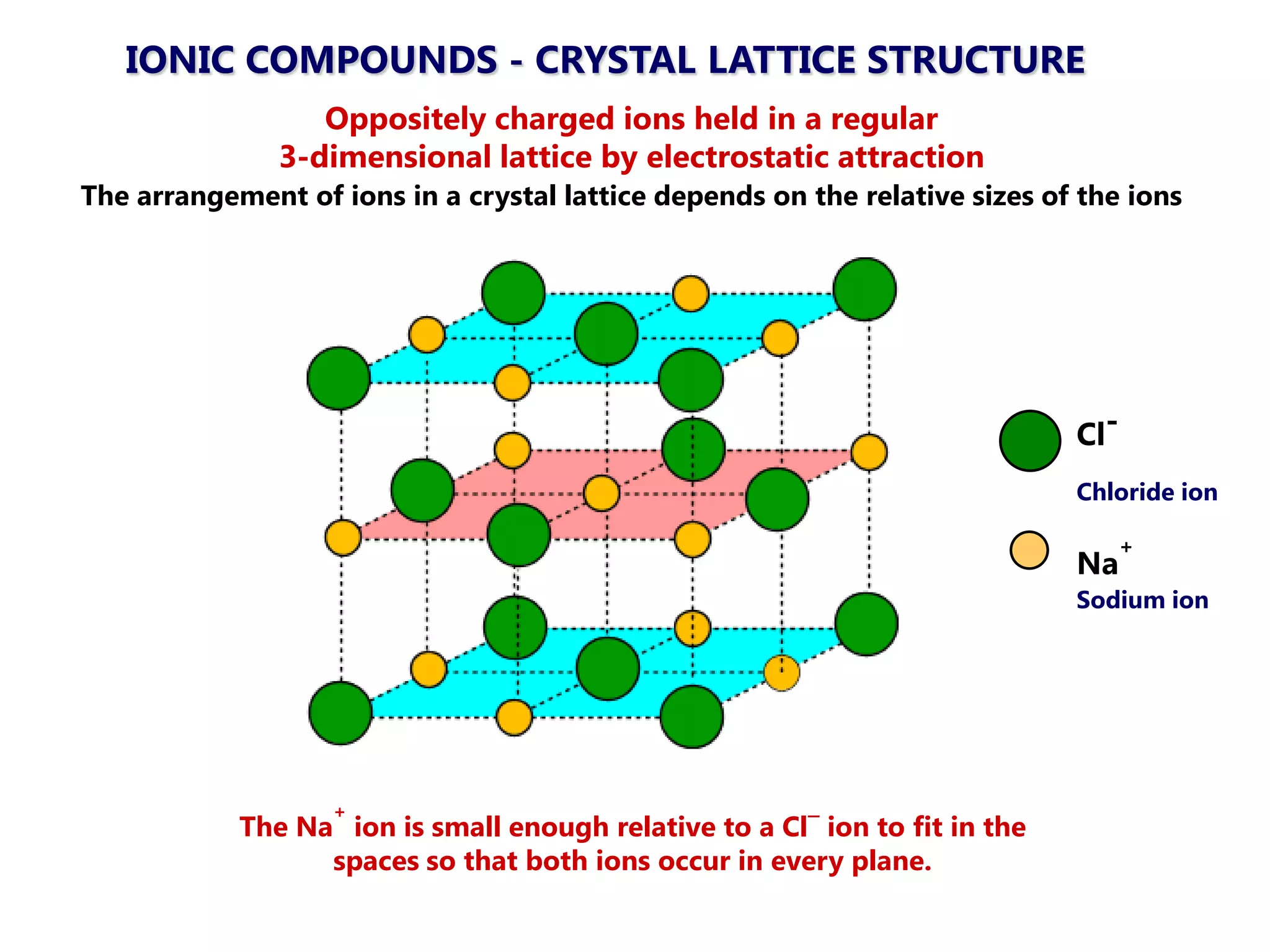
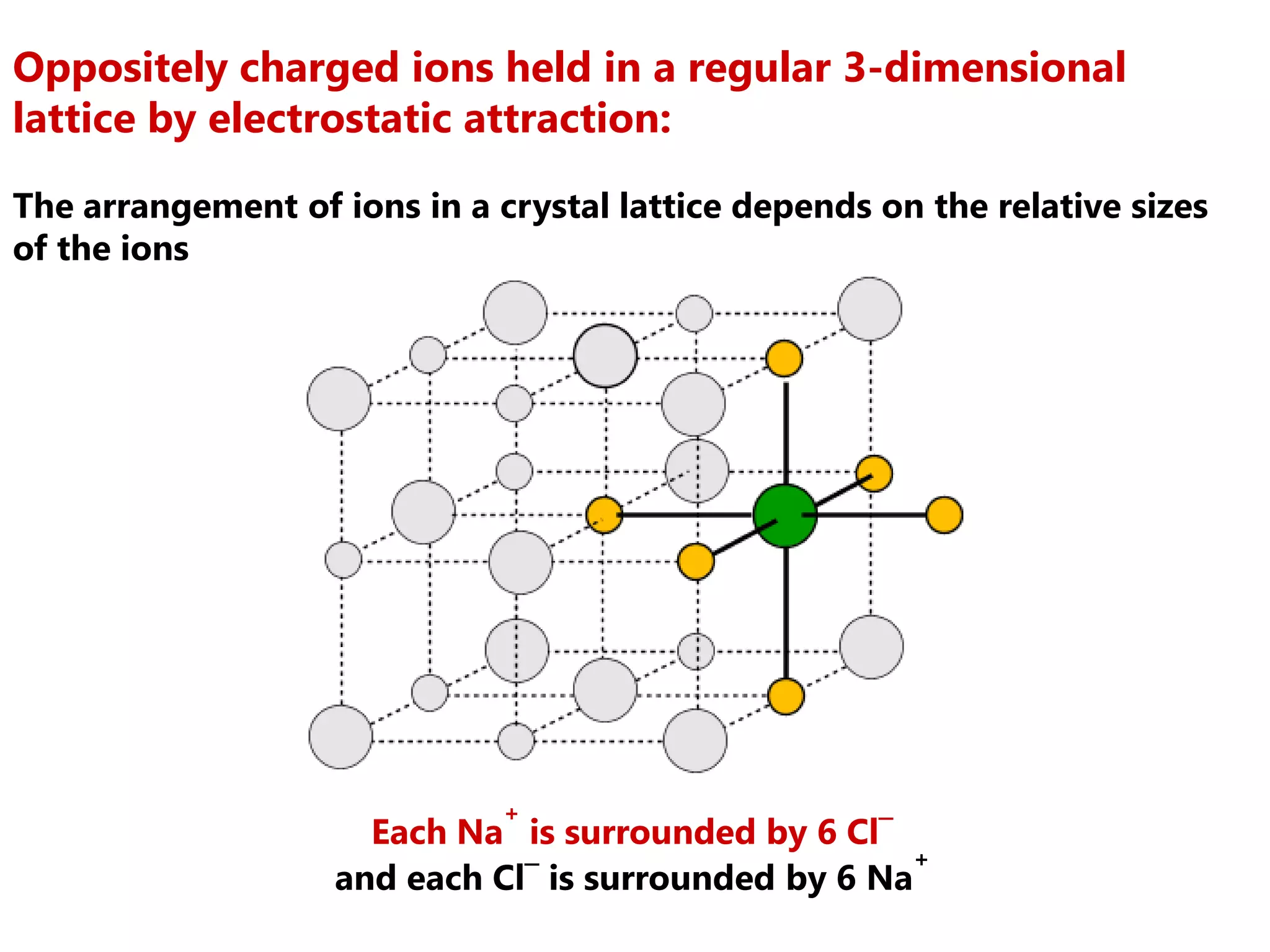
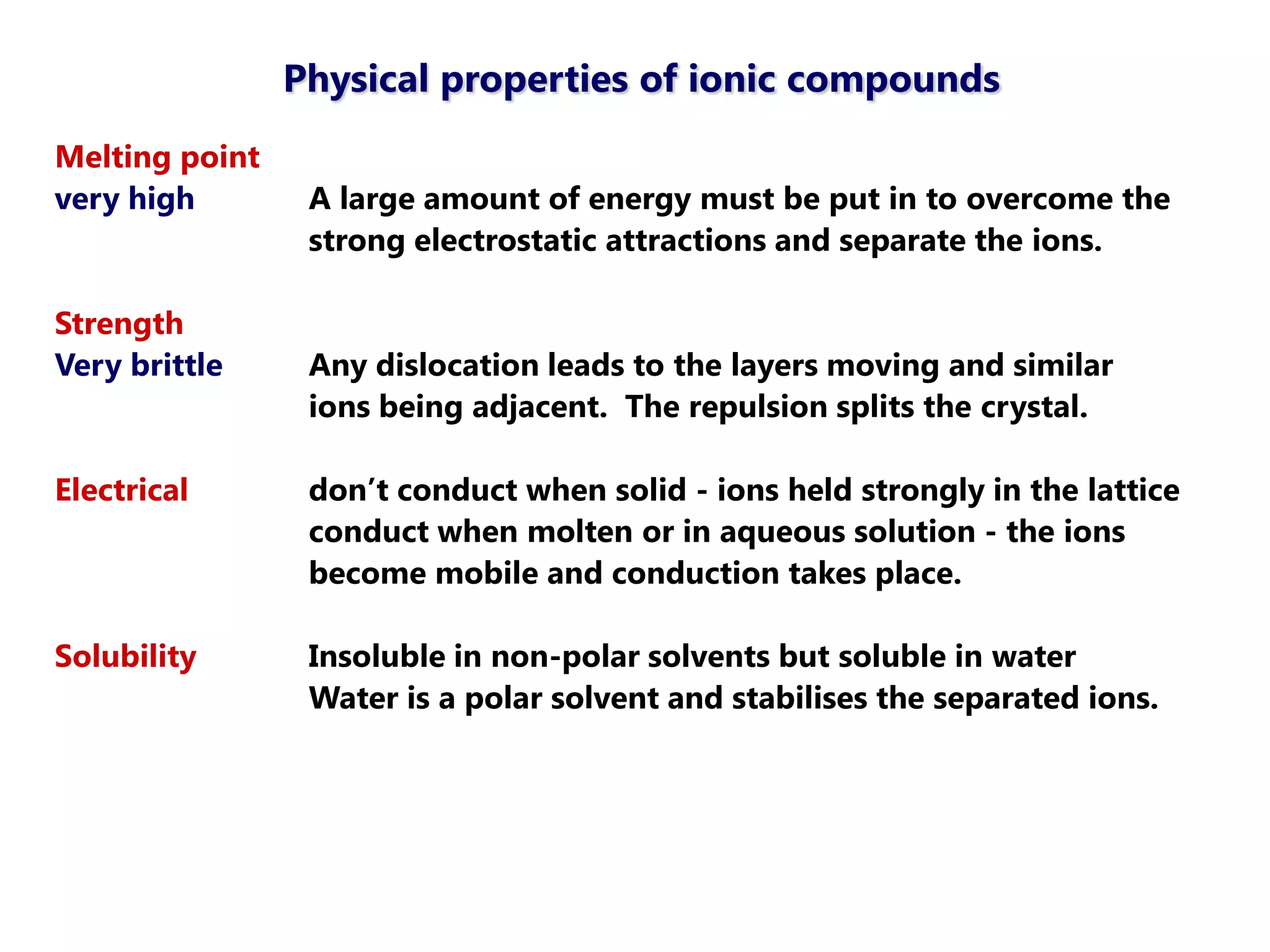
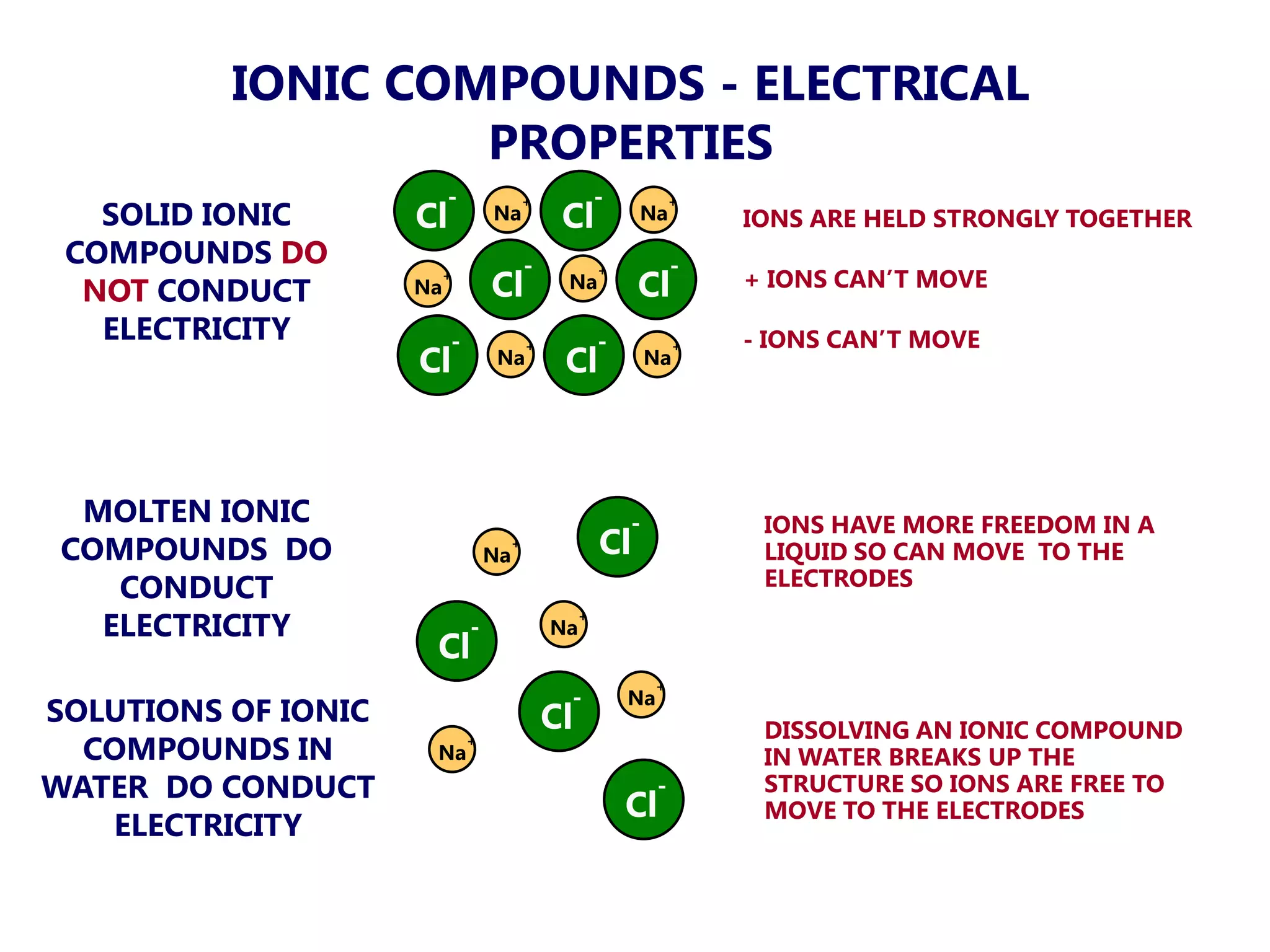
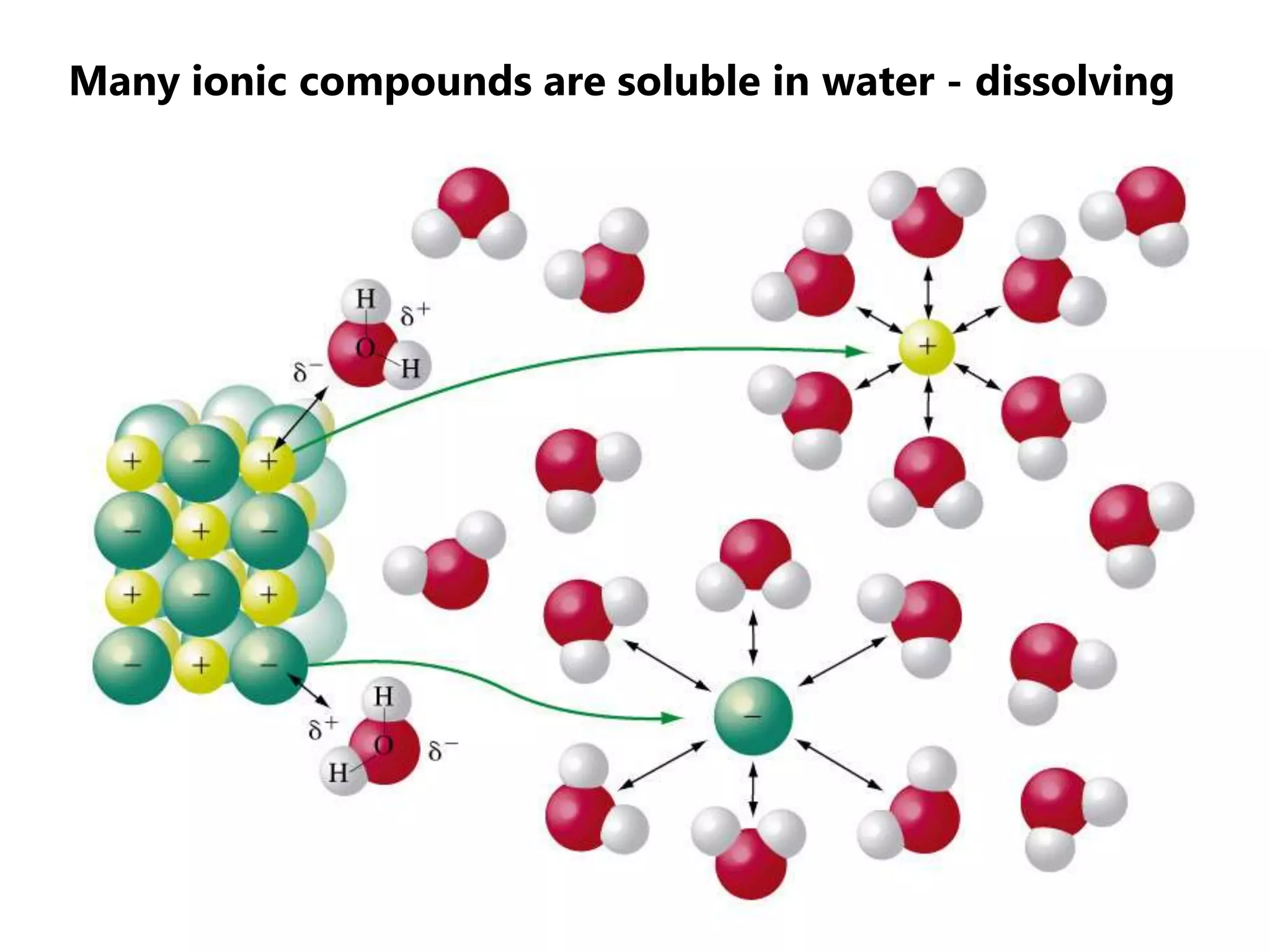
The document discusses ionic bonding. Ionic bonds form between elements when one atom loses electrons to become a positively charged cation and another atom gains those electrons to become a negatively charged anion. This transfer of electrons allows both atoms to achieve a stable noble gas electron configuration. The resulting ions are held together by electrostatic attraction in a crystal lattice structure. Ionic compounds have high melting points, are brittle, and do not conduct electricity as solids since the ions are tightly bound. They dissolve in water, allowing the ions to separate and move, making the solutions electrically conductive.