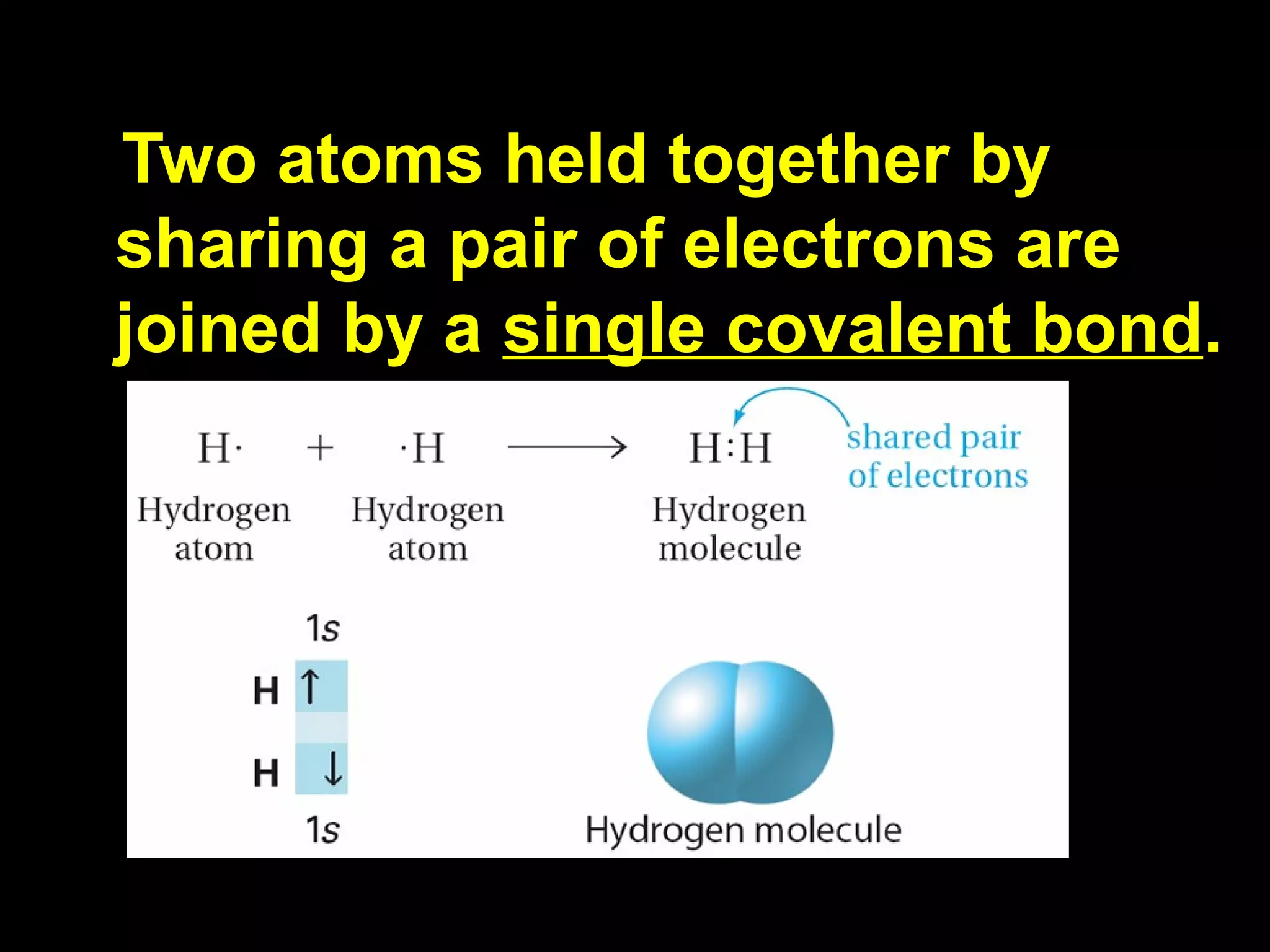
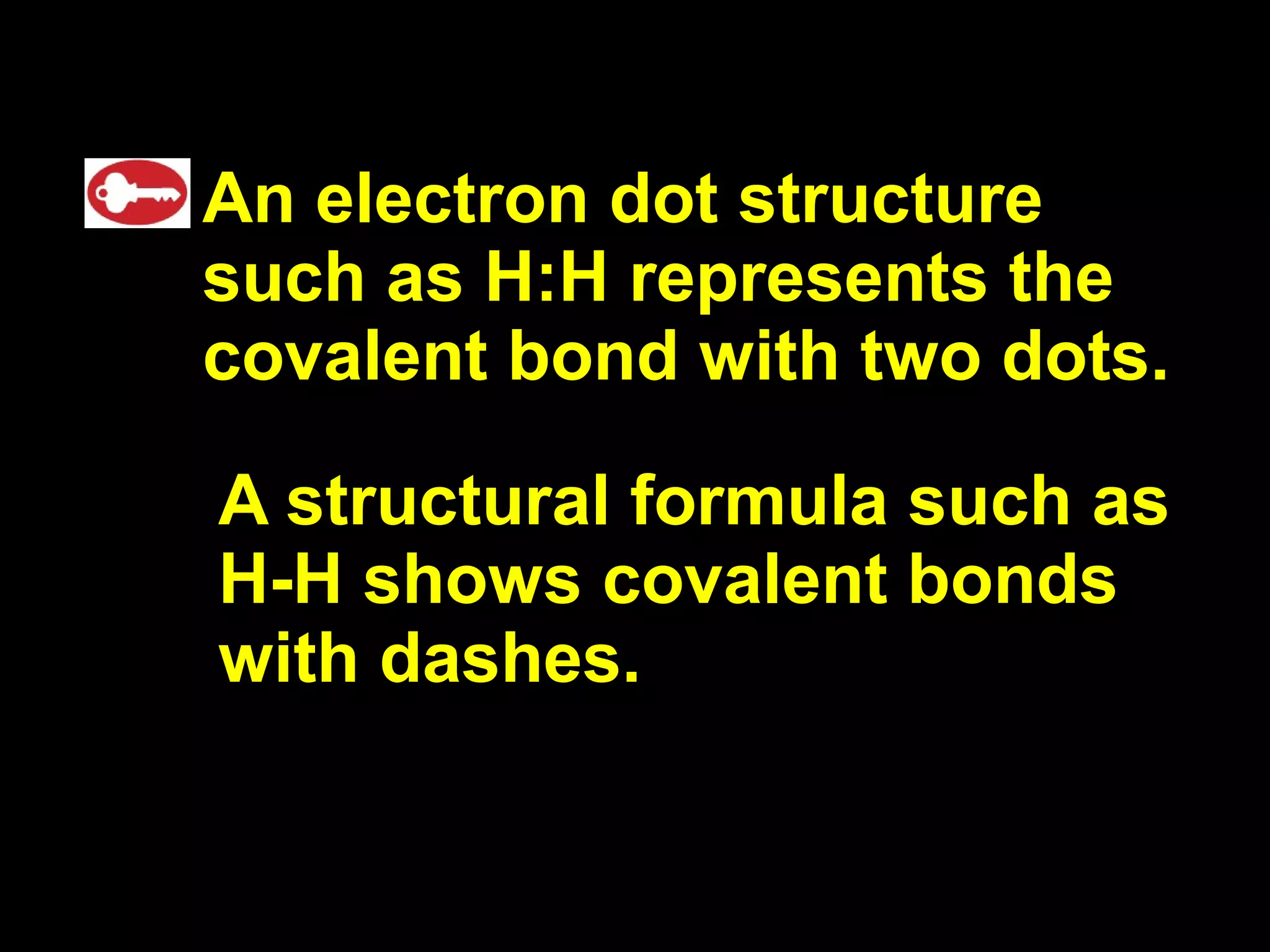
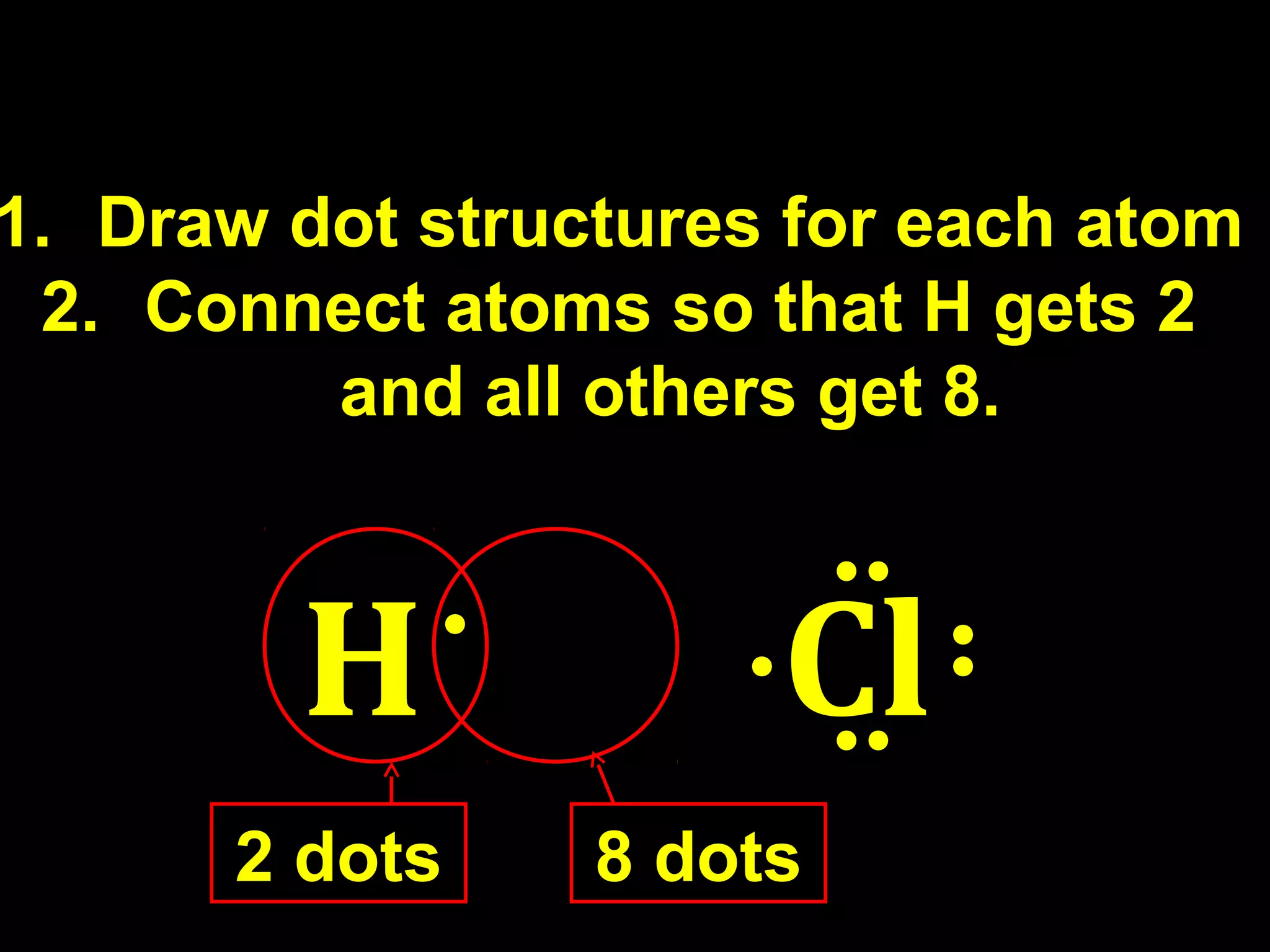
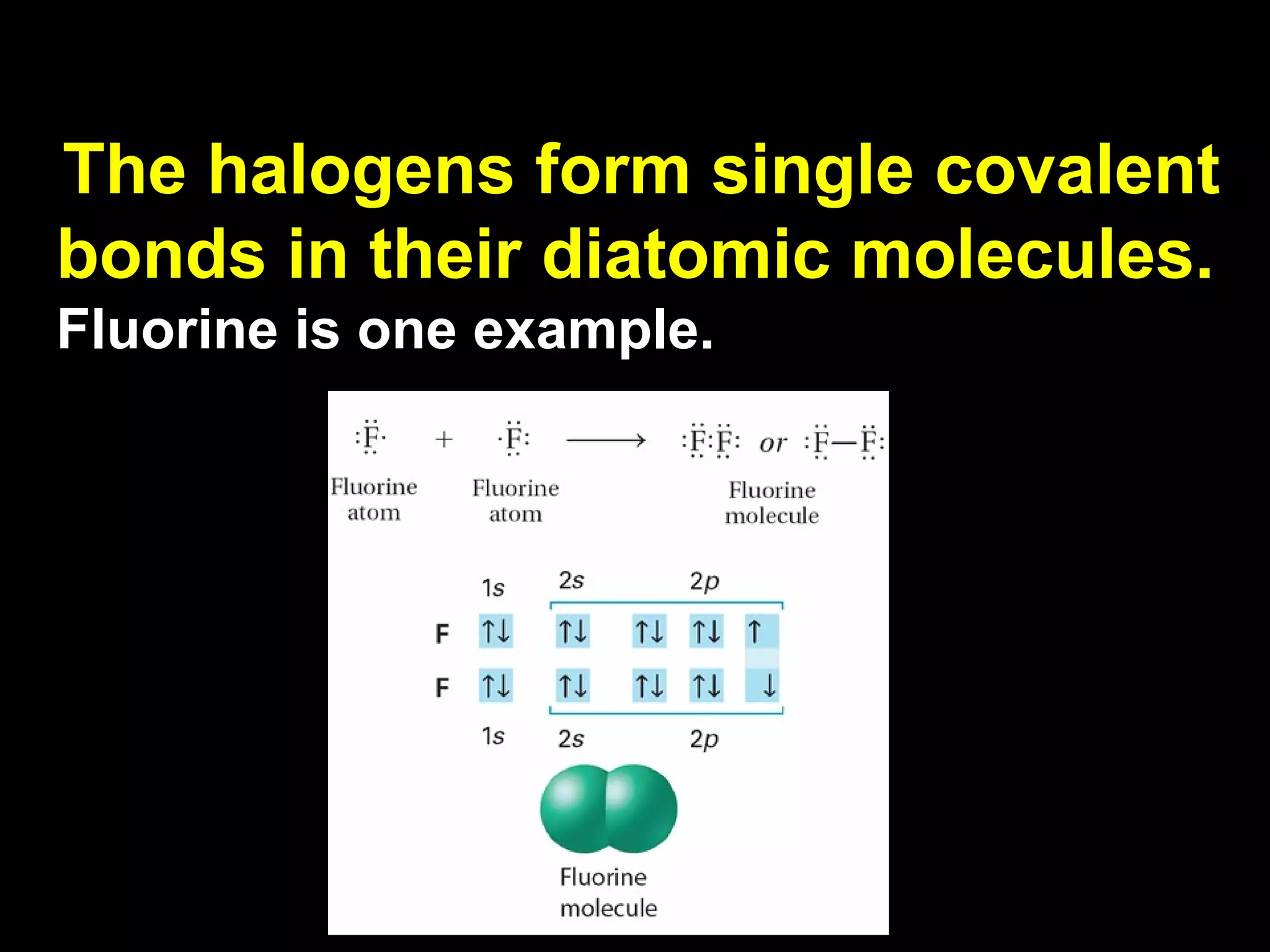
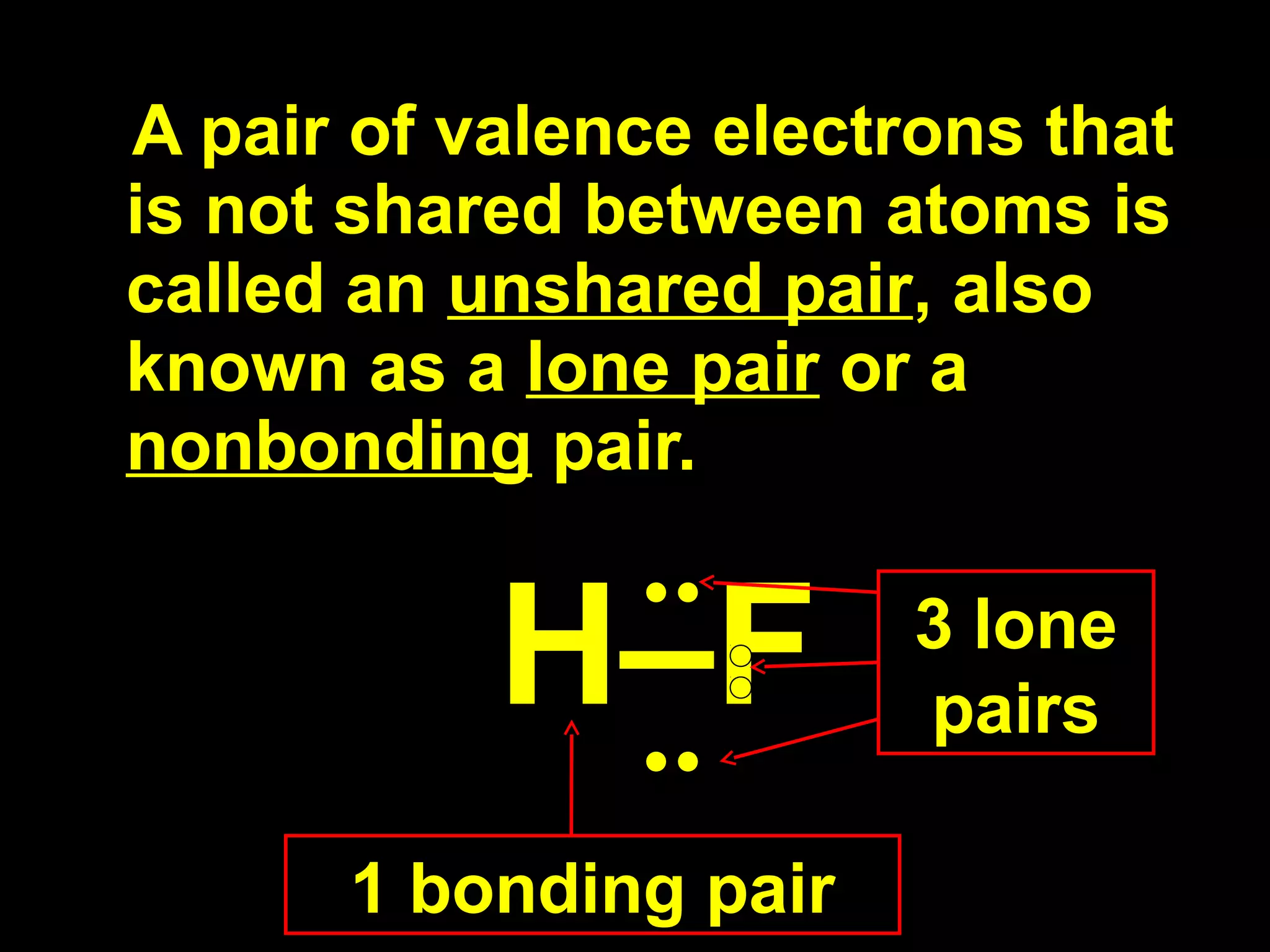
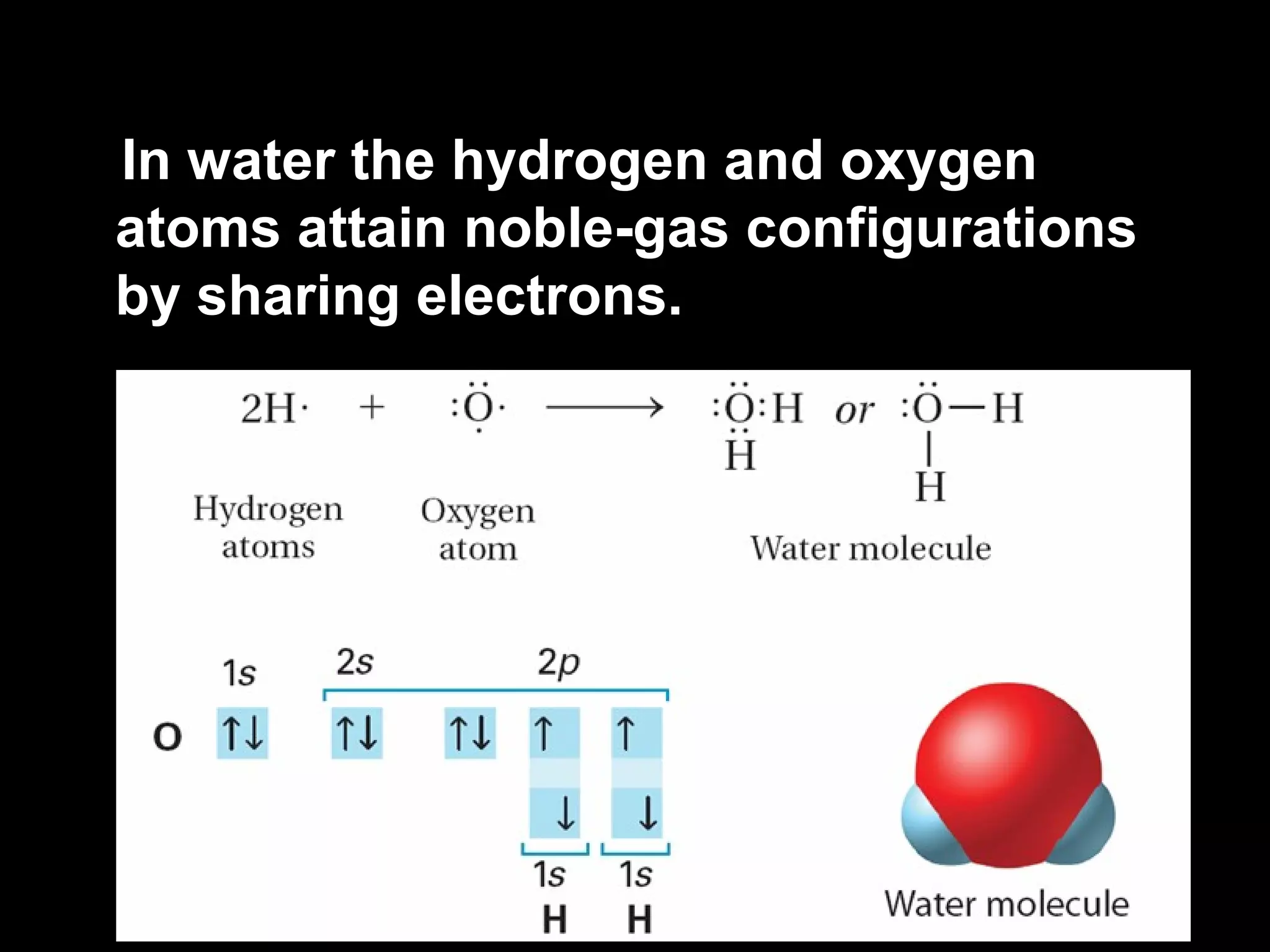
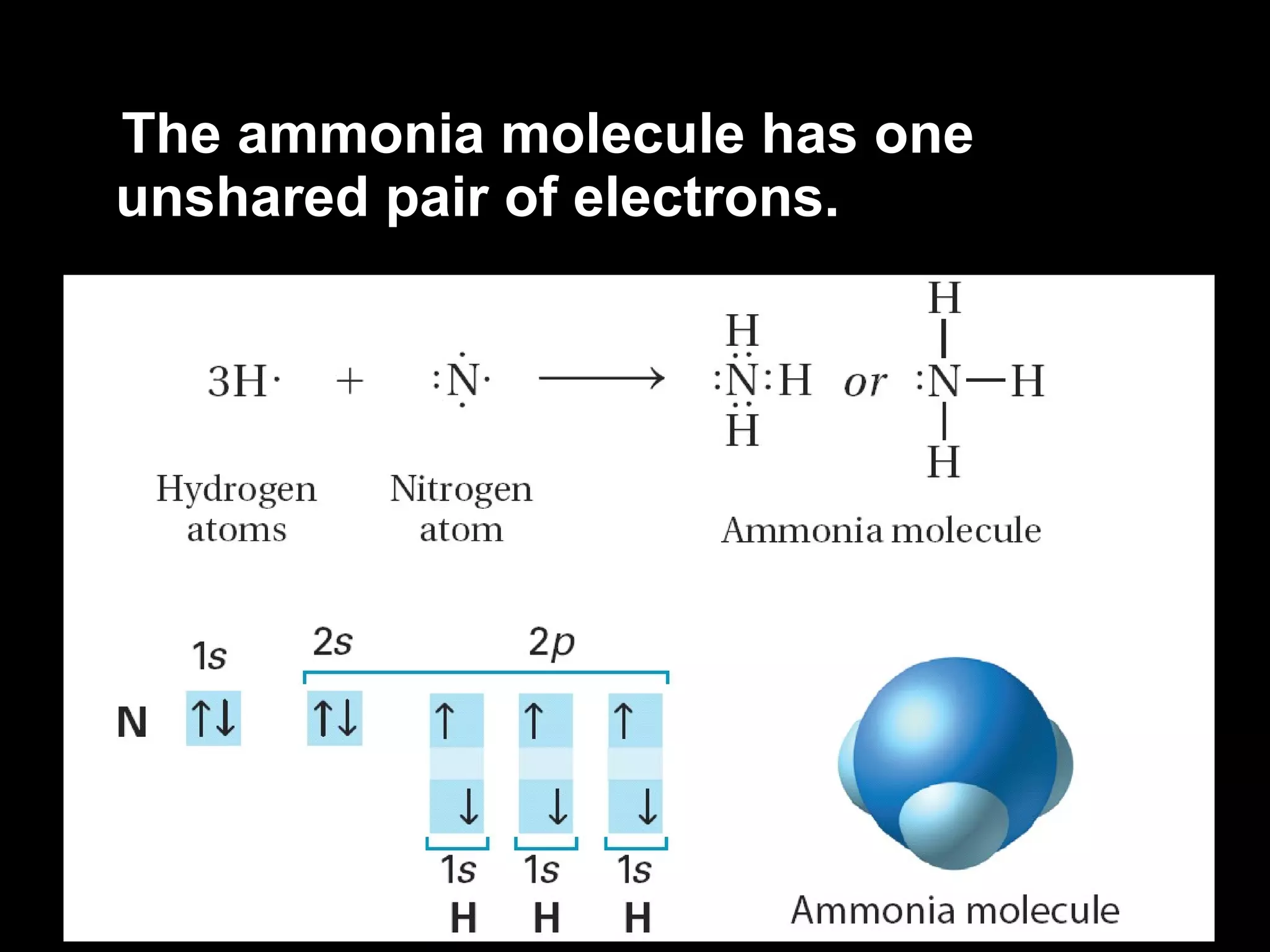
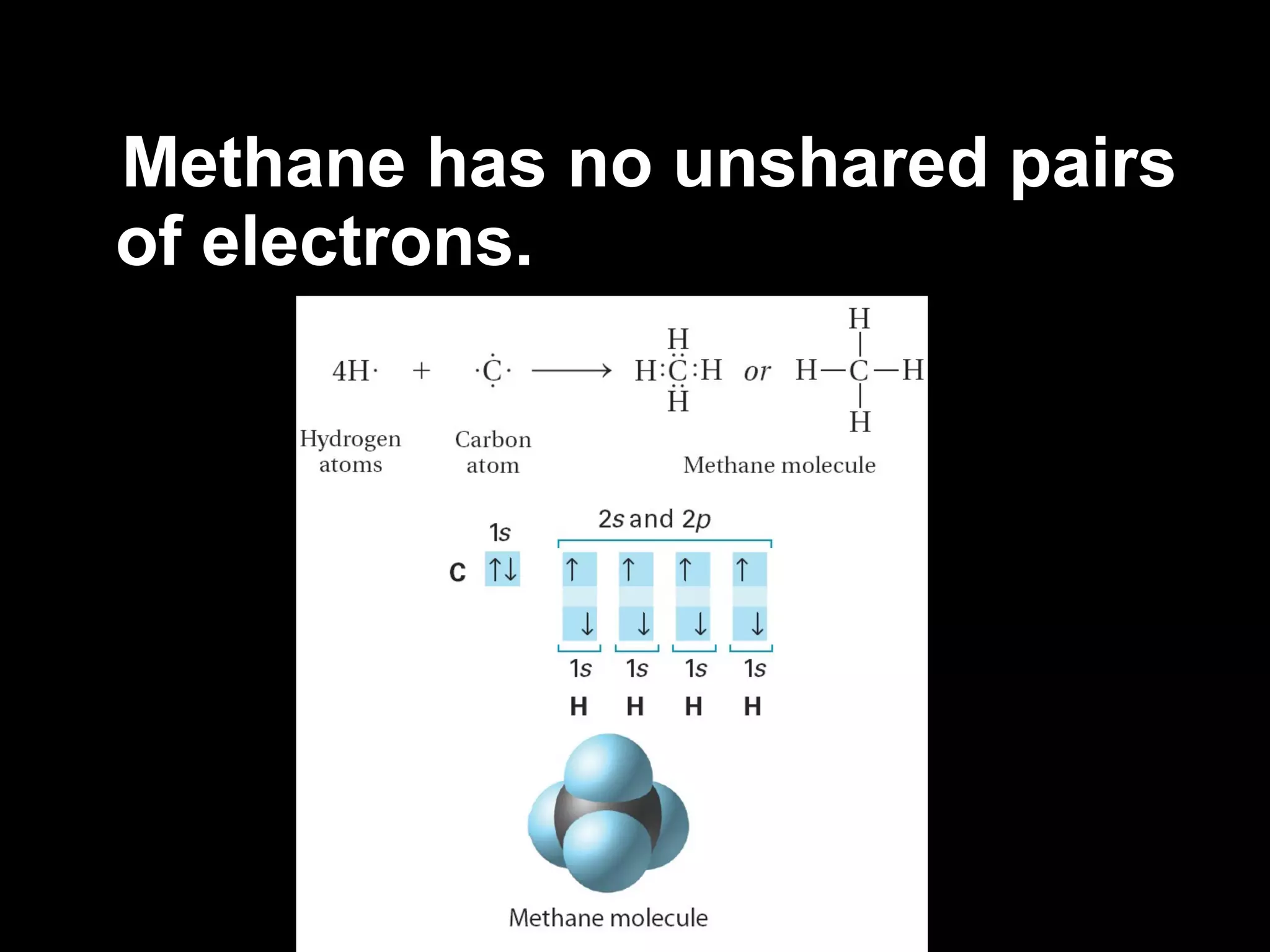
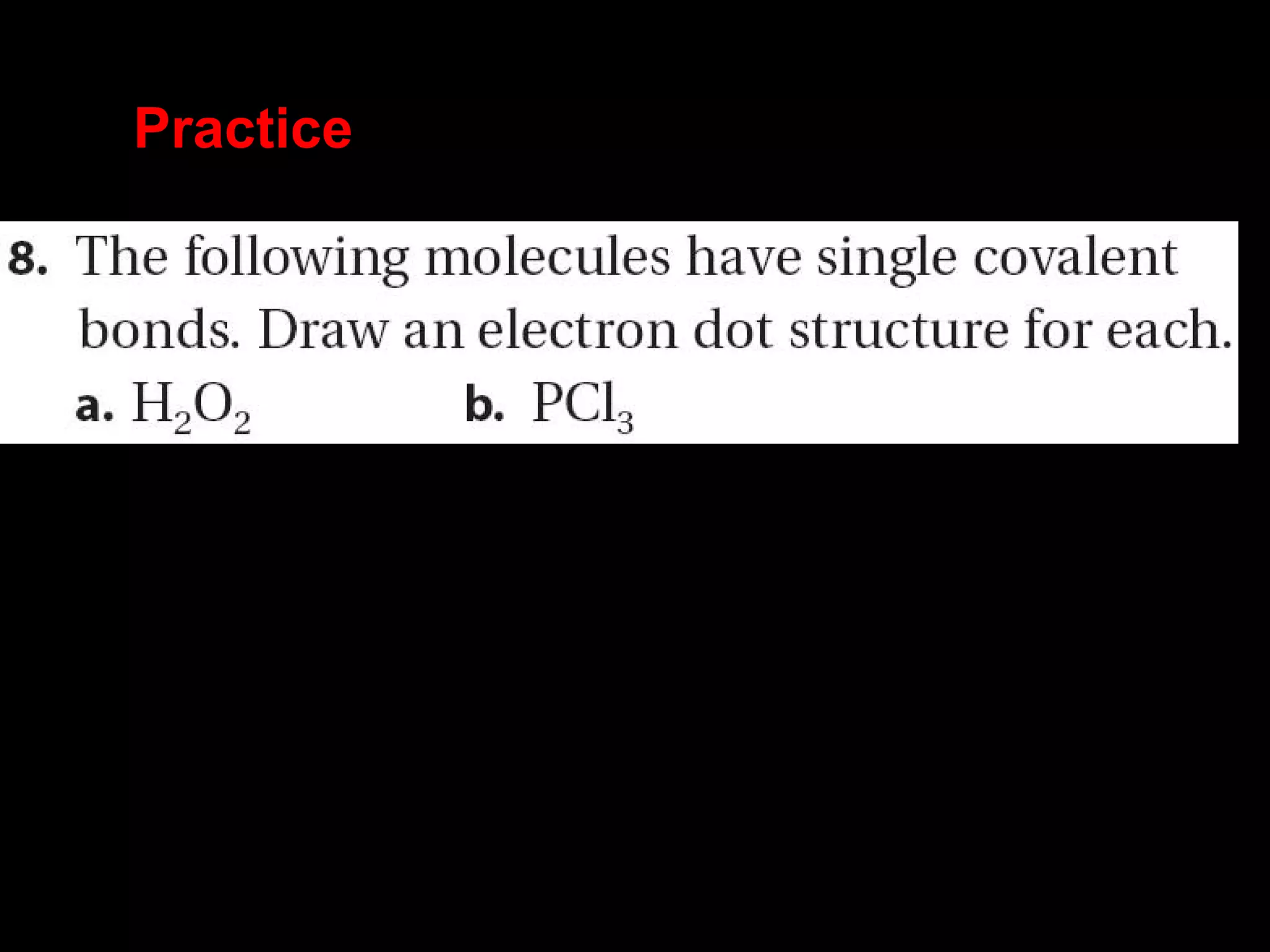
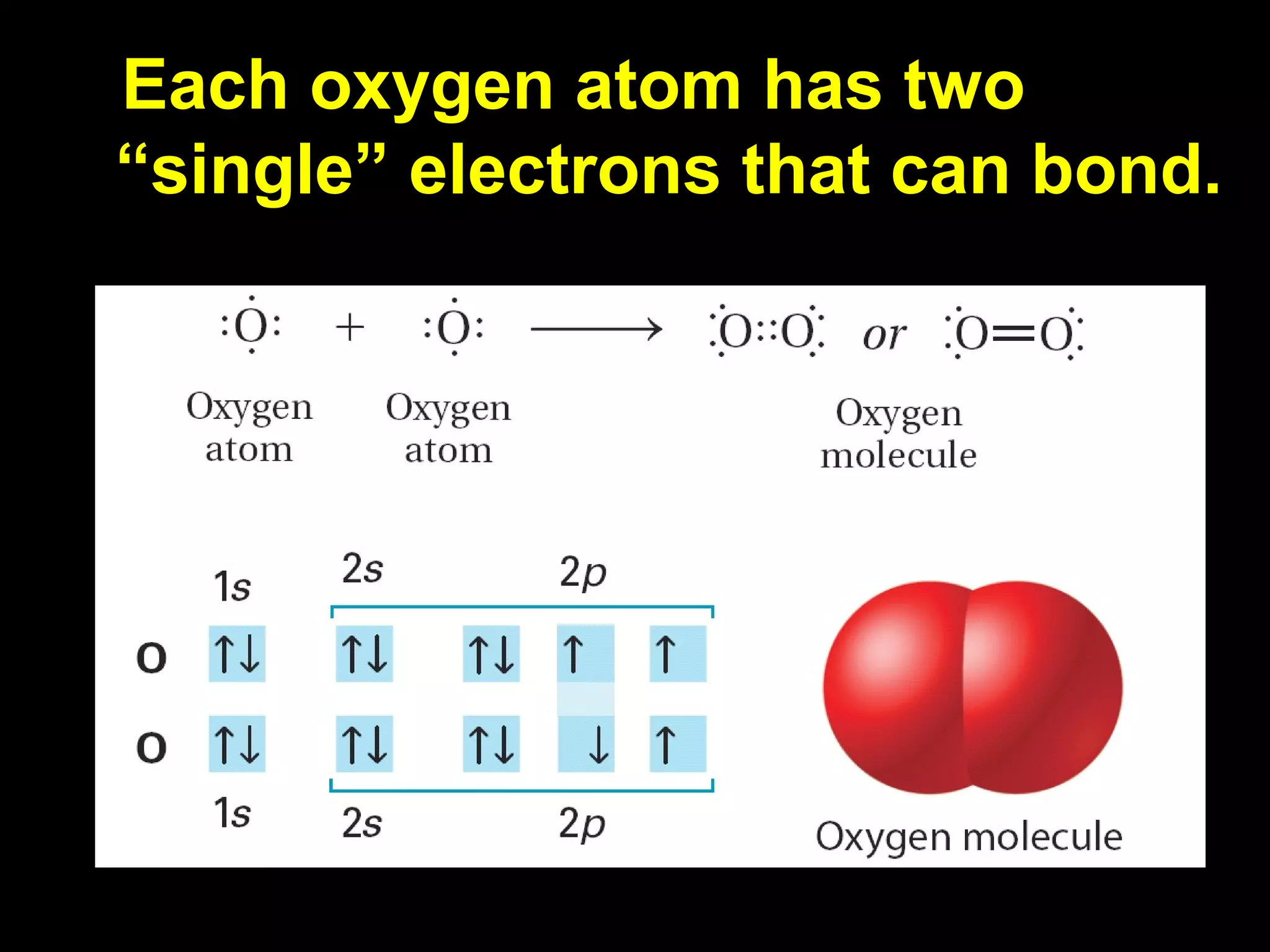
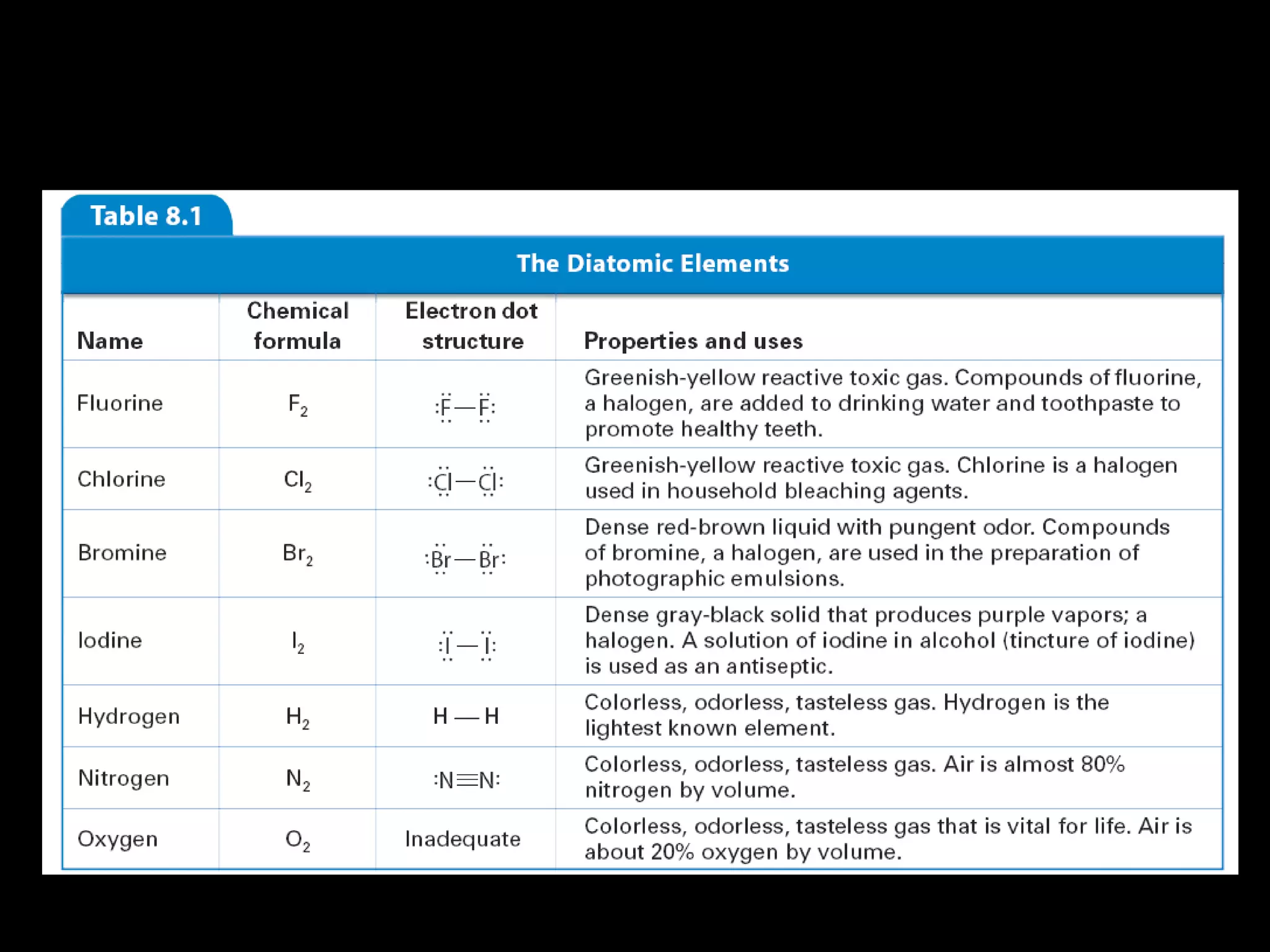
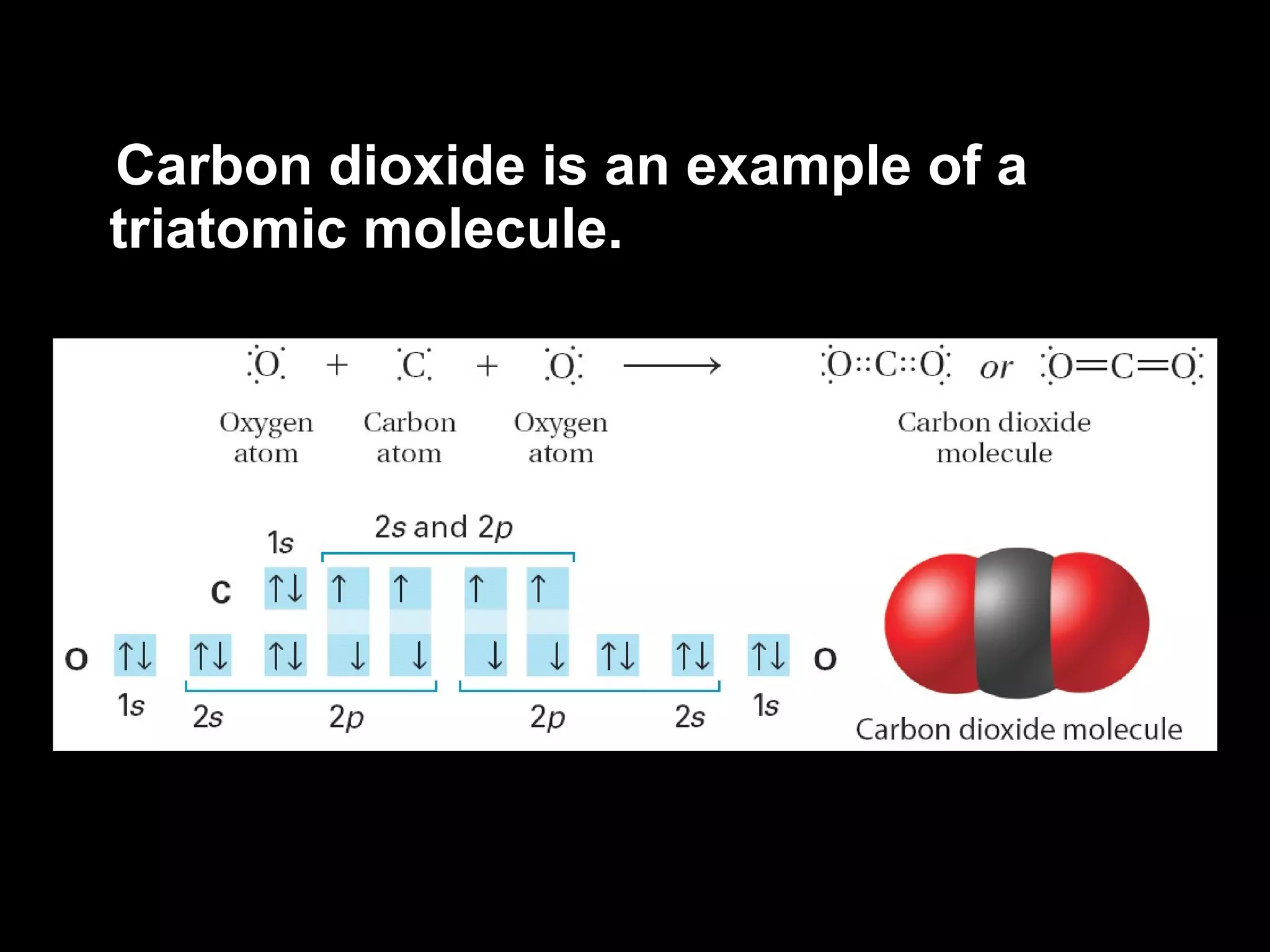
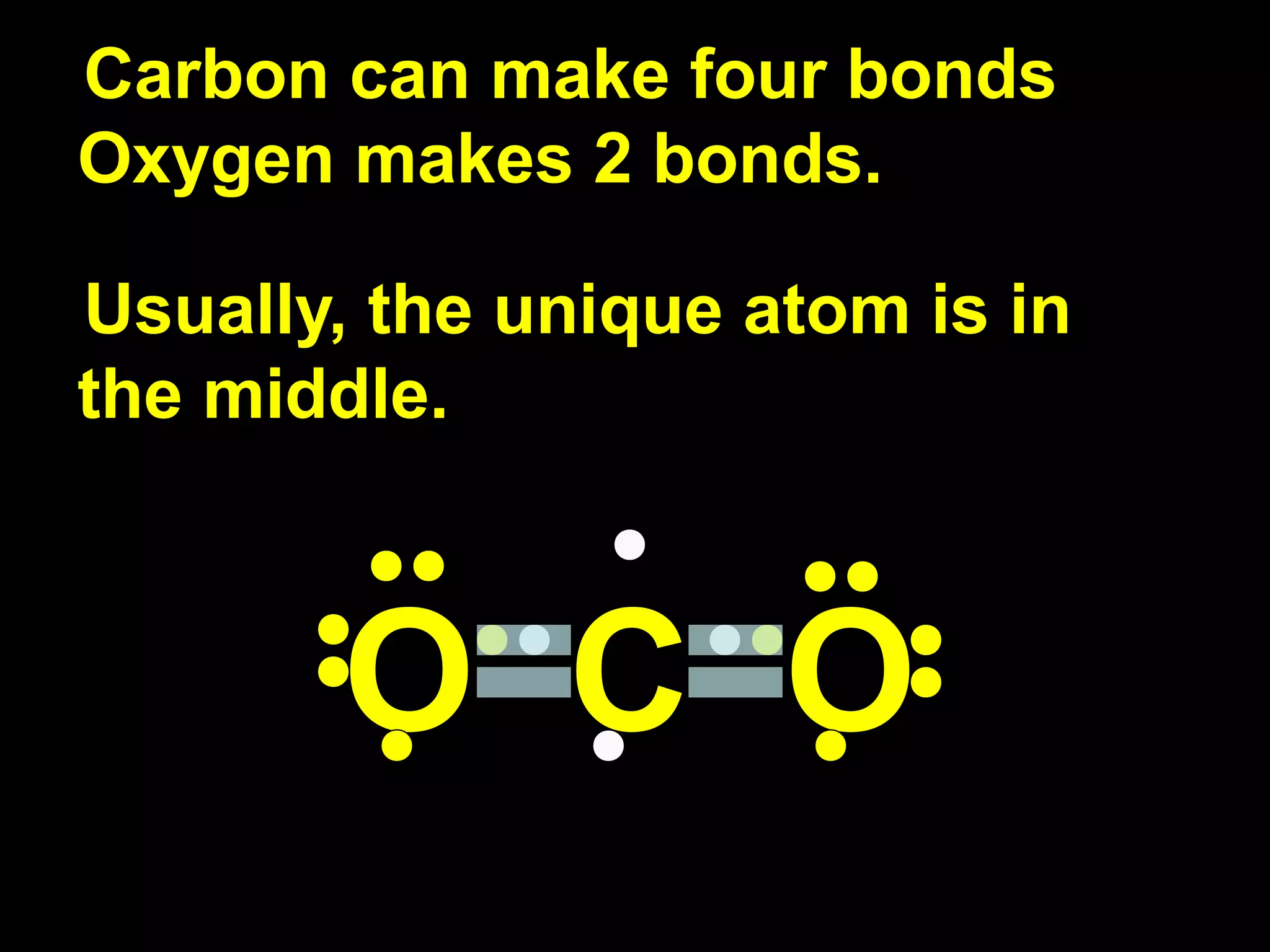
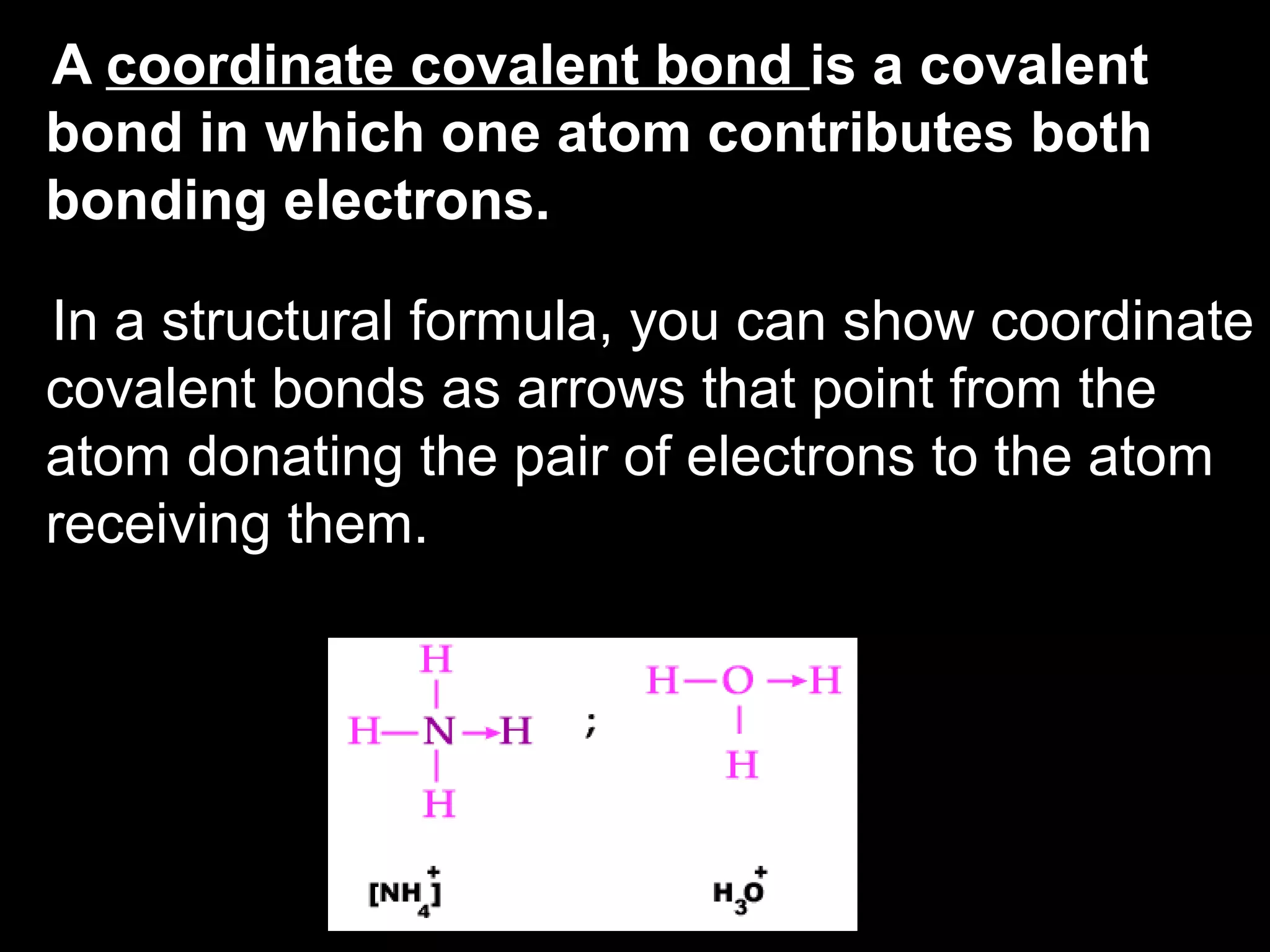
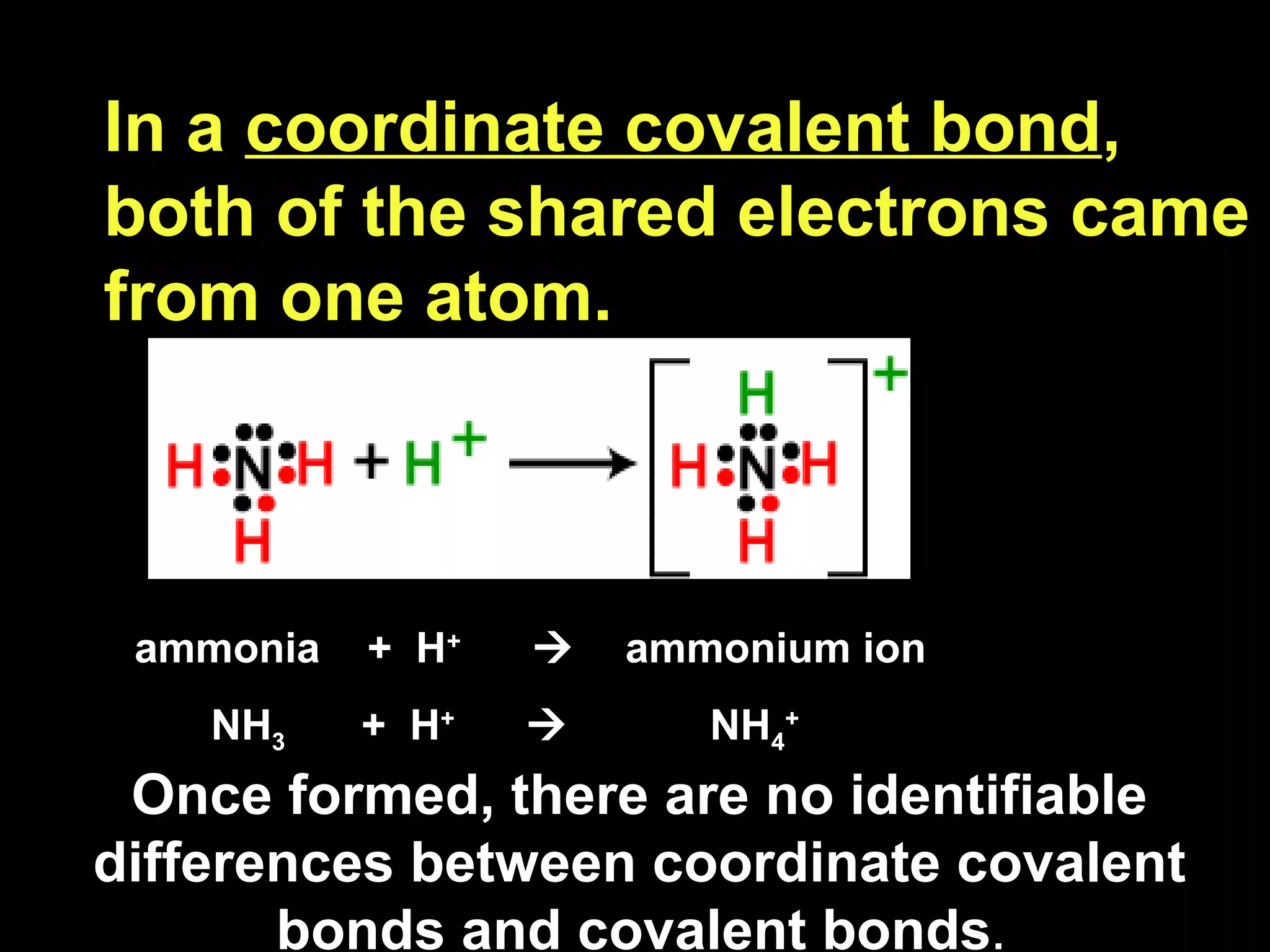
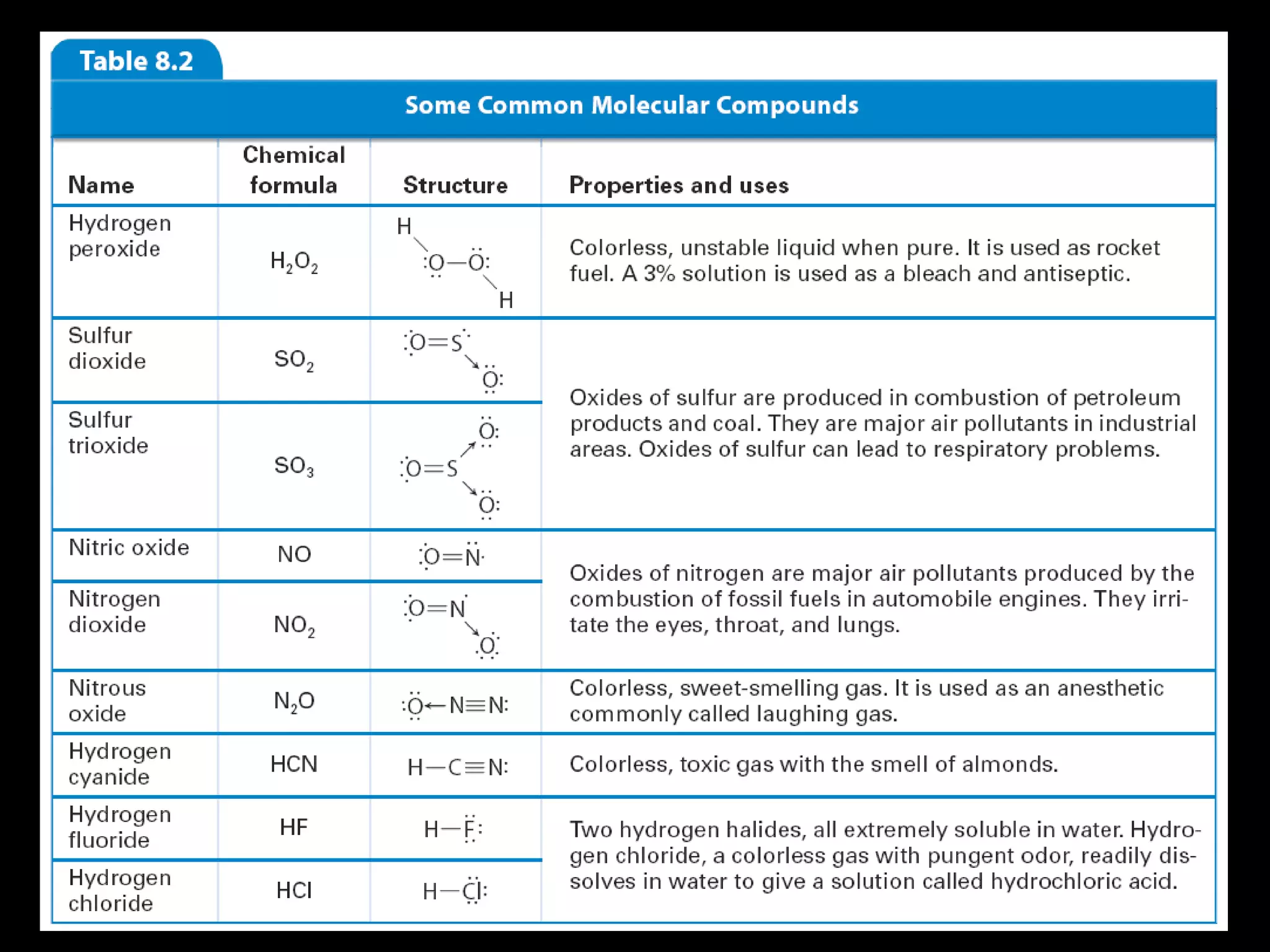
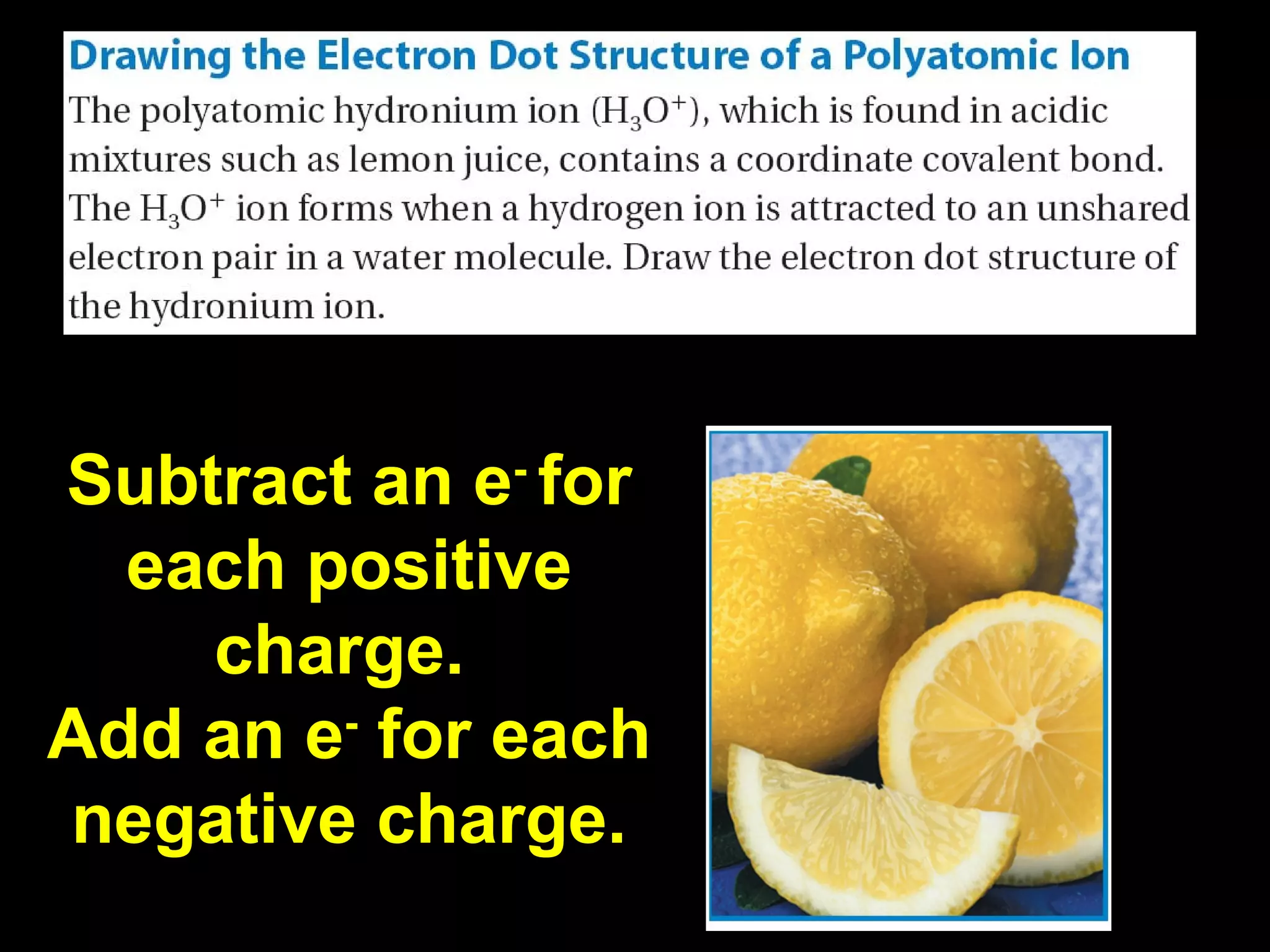
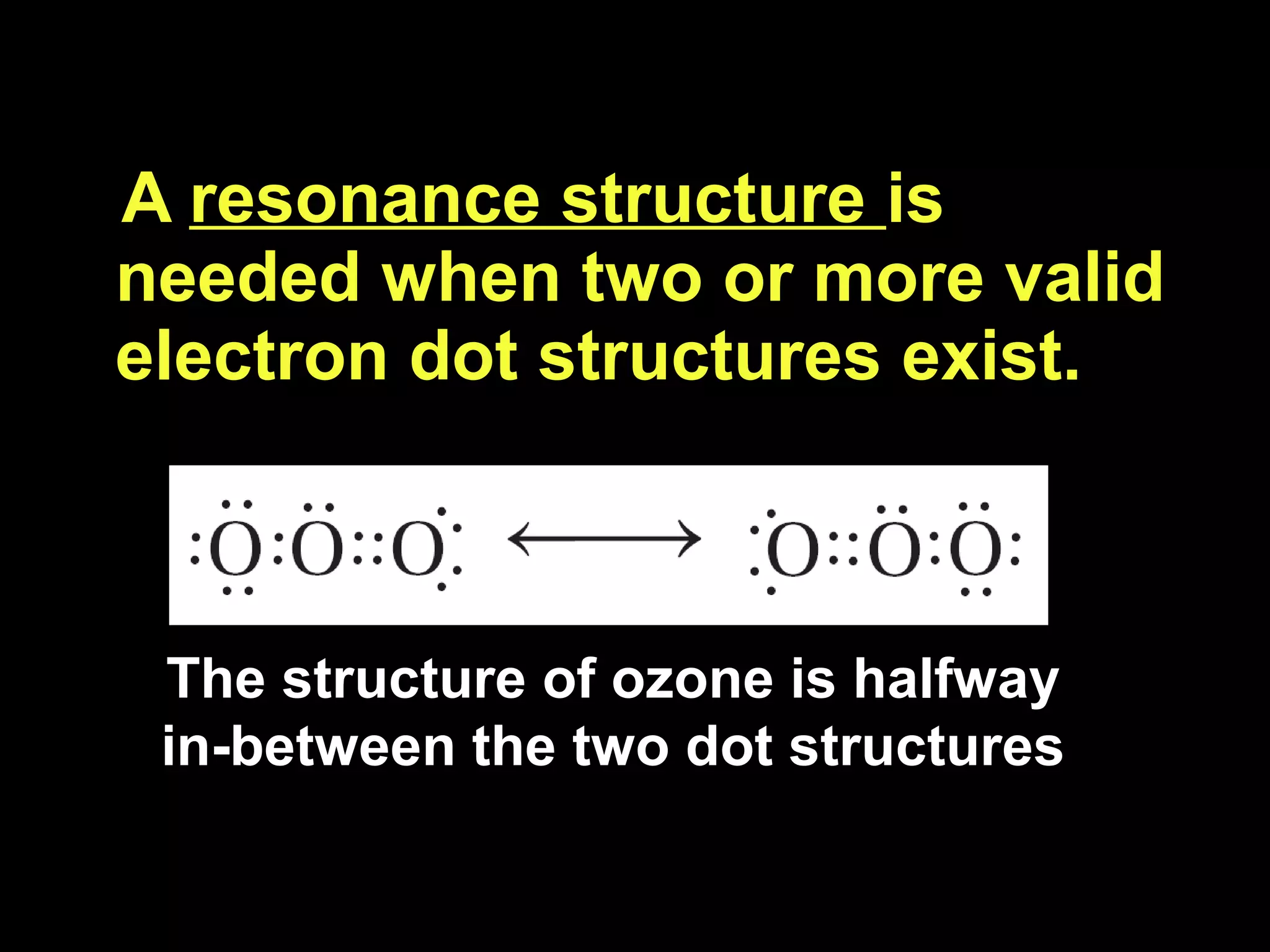
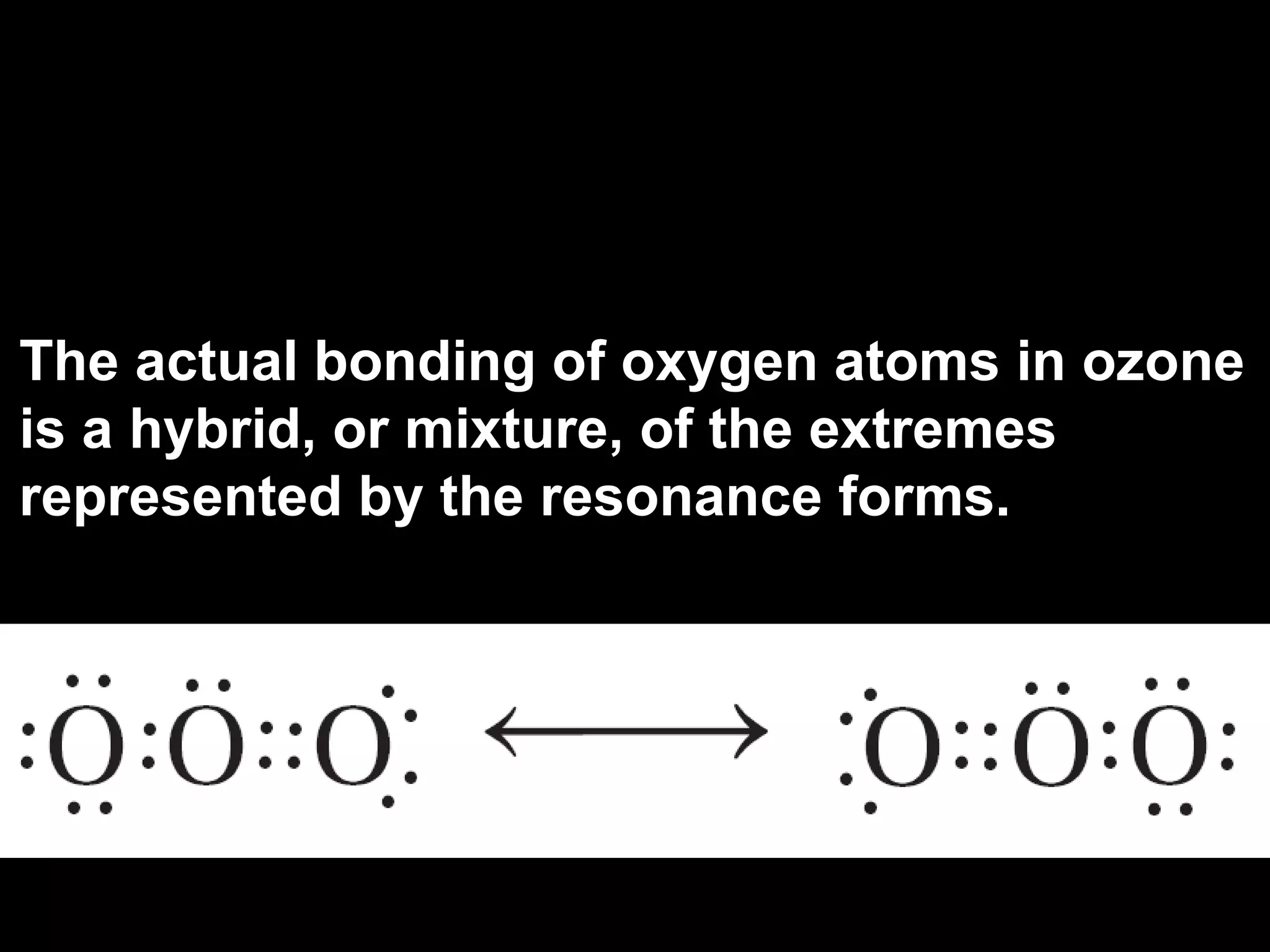
The document discusses ionic and covalent bonding. It explains how to draw Lewis dot structures to show electron sharing between atoms to form single, double or triple covalent bonds. Examples are given of molecules like H2O, NH3, CH4, CO2, and O3 that form different types of covalent bonds through electron sharing.