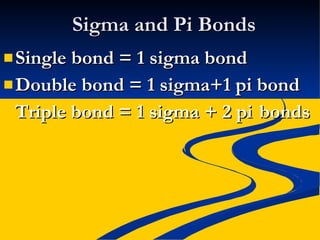
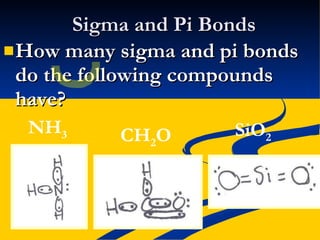
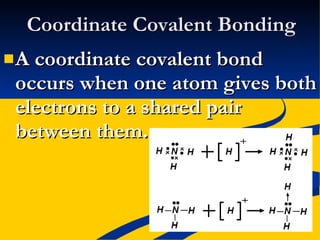
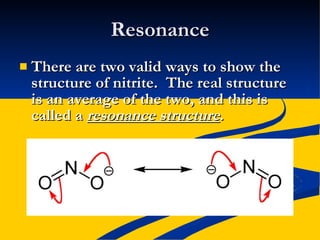
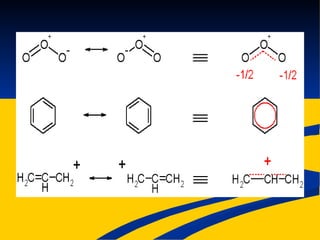
This document provides notes on covalent bonding concepts including:
- The octet rule and how atoms form bonds to gain or lose electrons
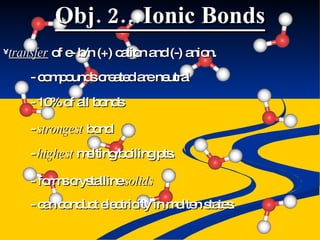
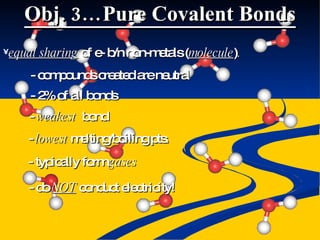
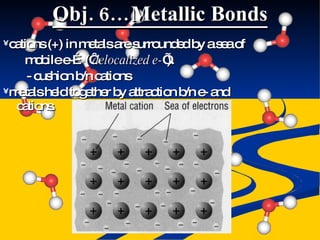
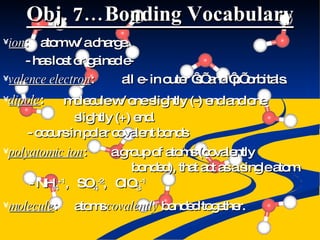
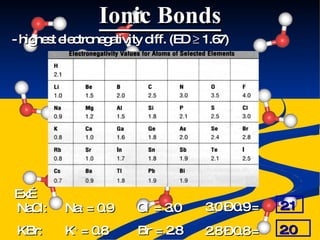
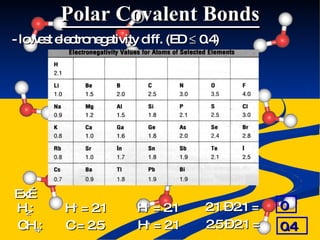
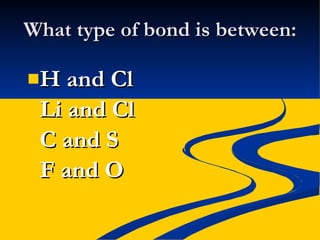
- Ionic, covalent, and polar covalent bonds defined by electron transfer and sharing
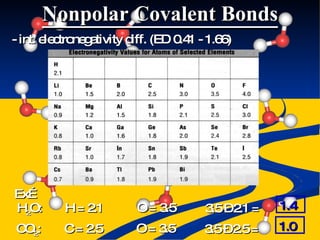
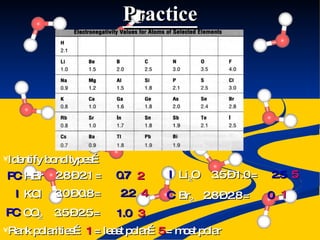
- Bond polarity determined by electronegativity differences
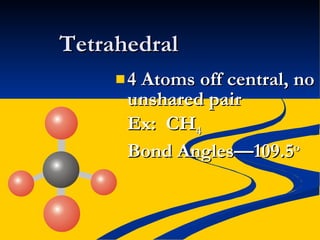
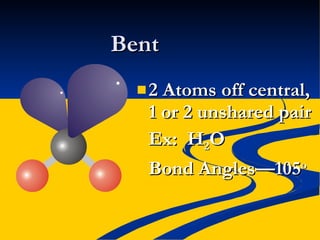
- Molecular polarity based on bond polarity and molecular geometry
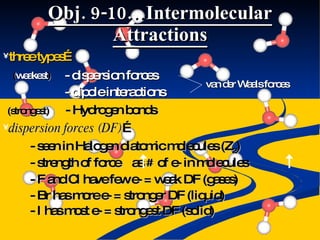
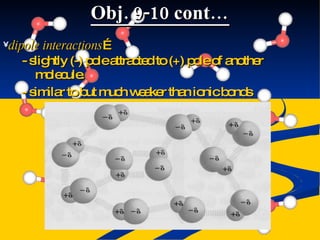
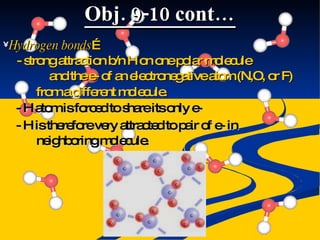
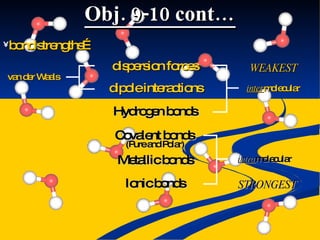
- Intermolecular forces of dispersion, dipole, and hydrogen bonding explained