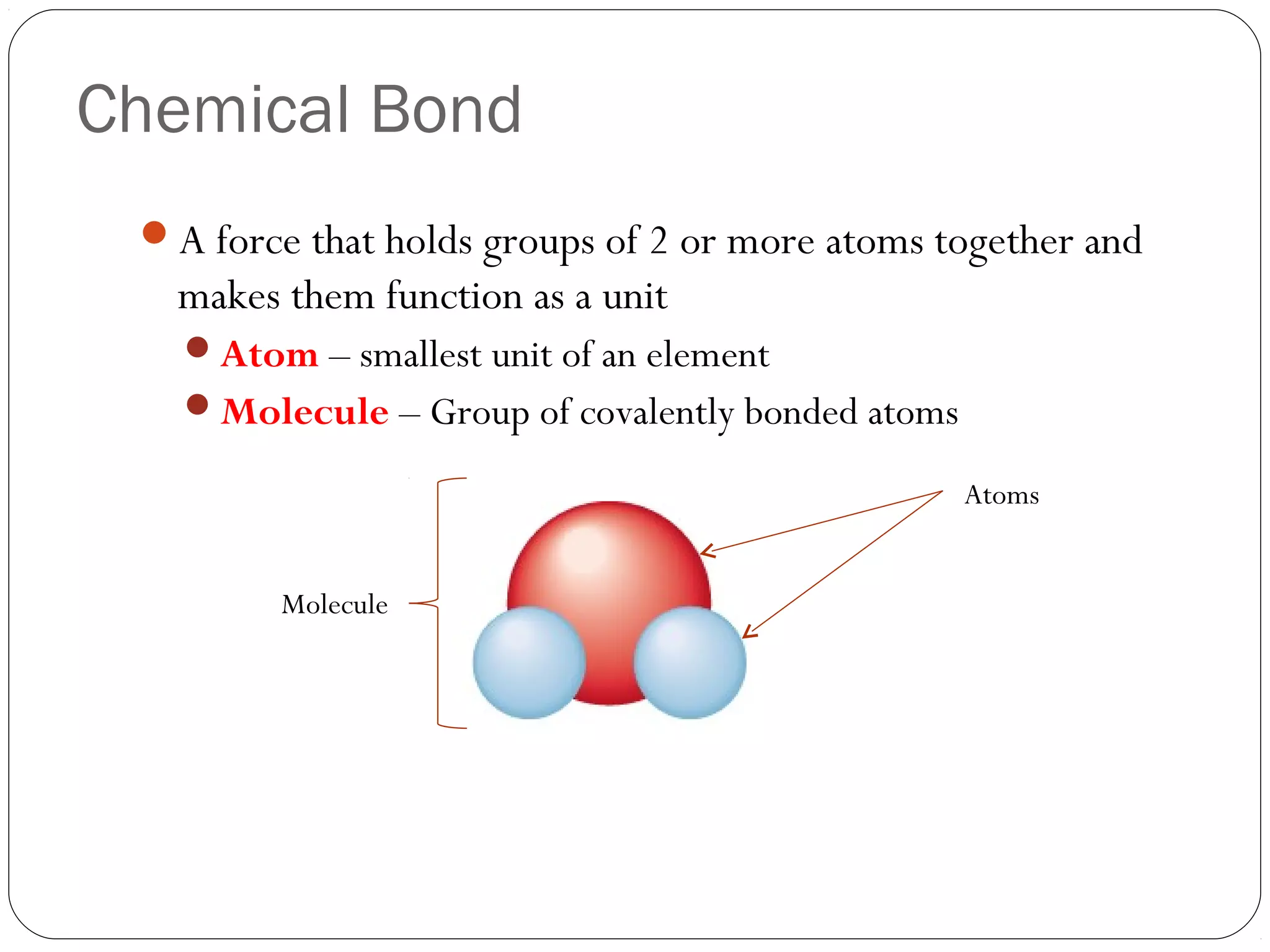
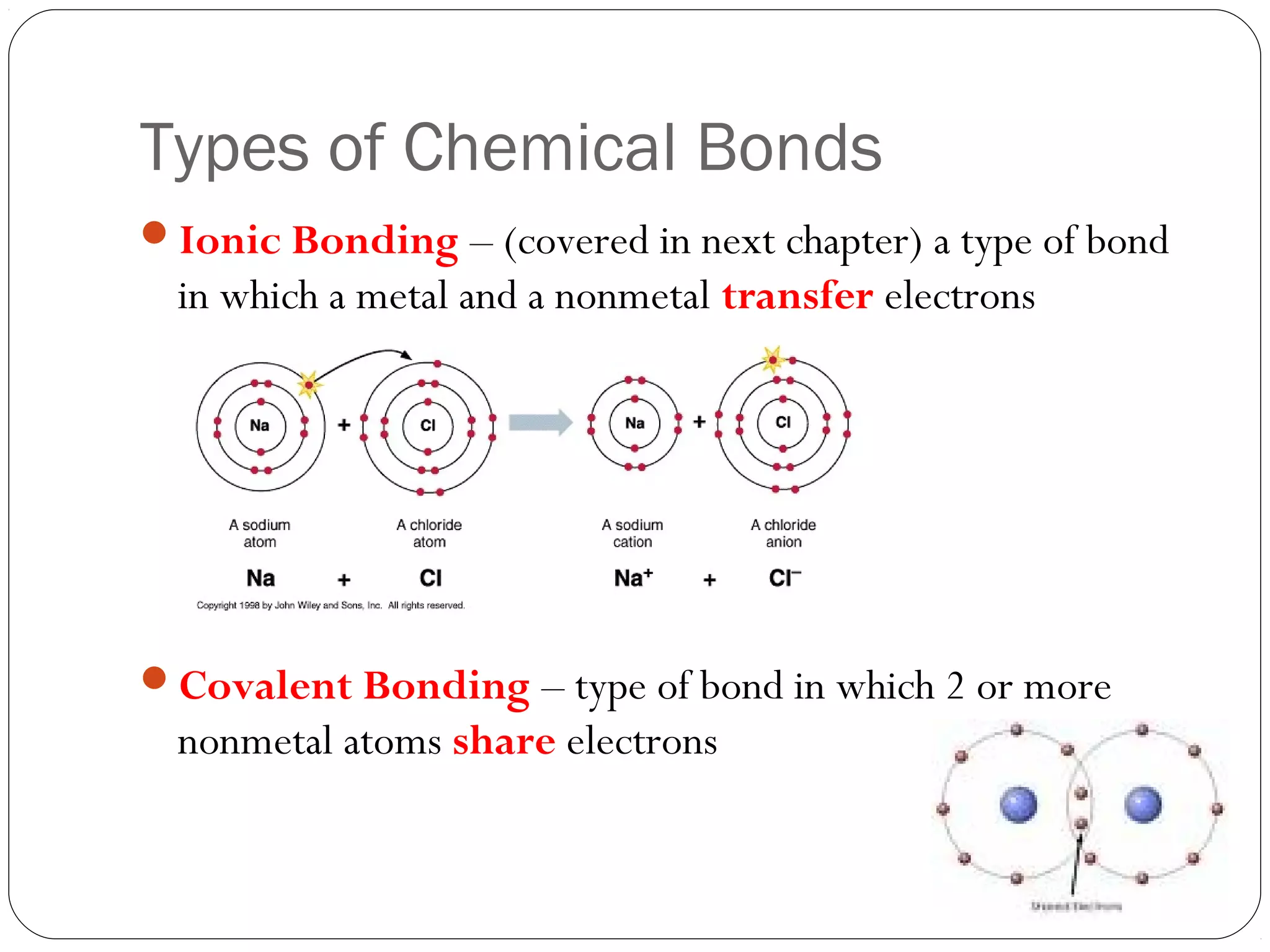
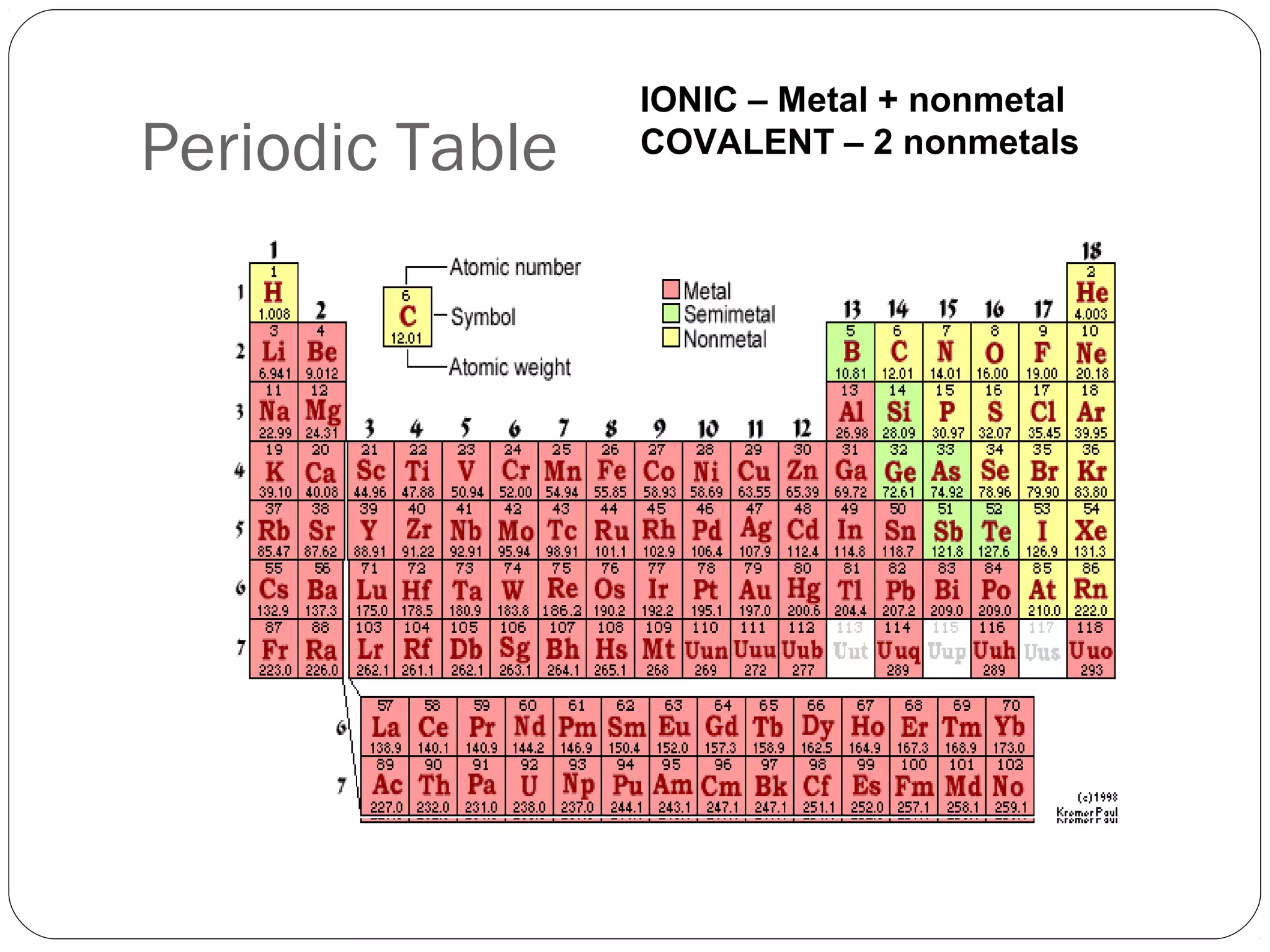
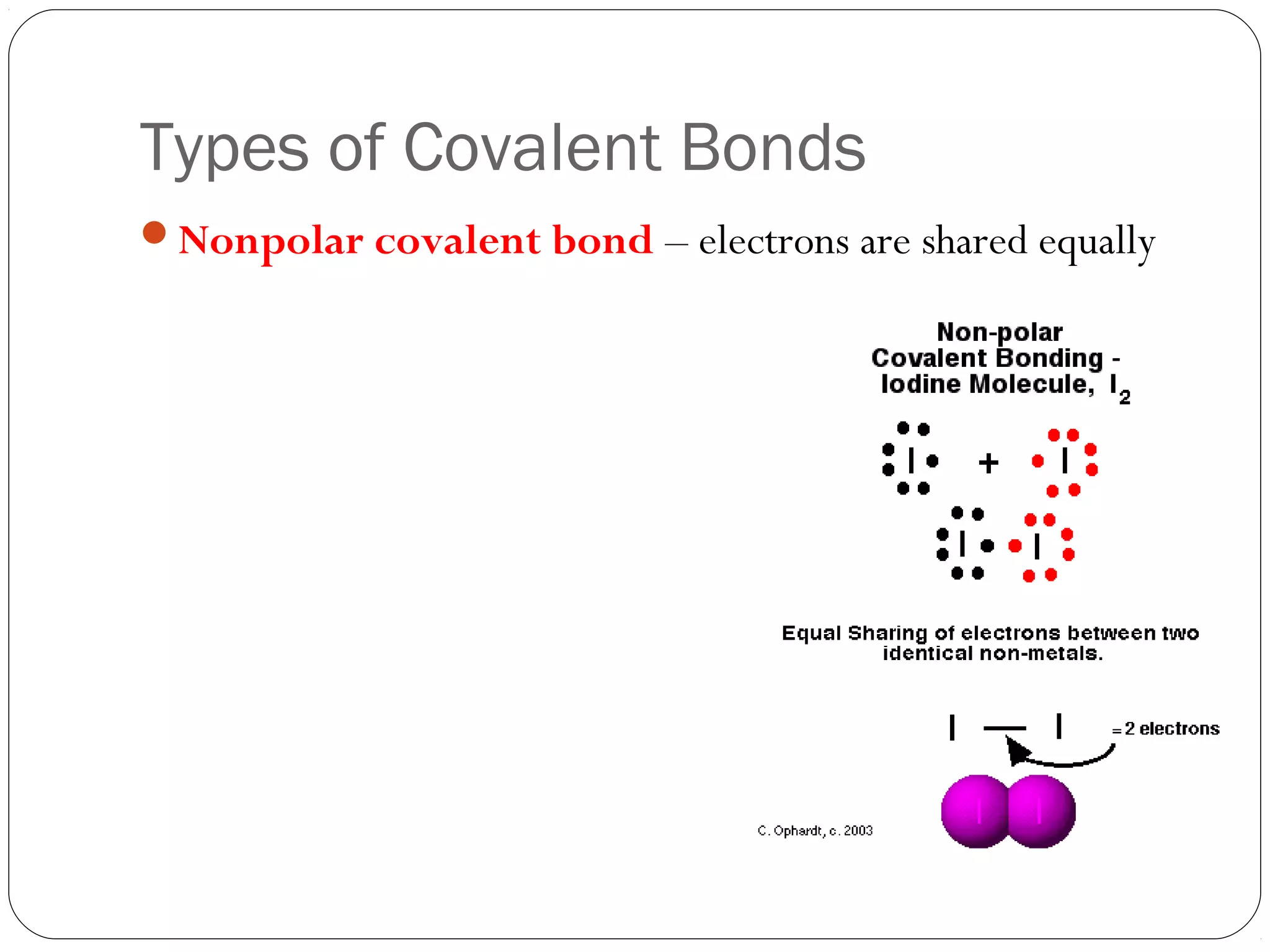
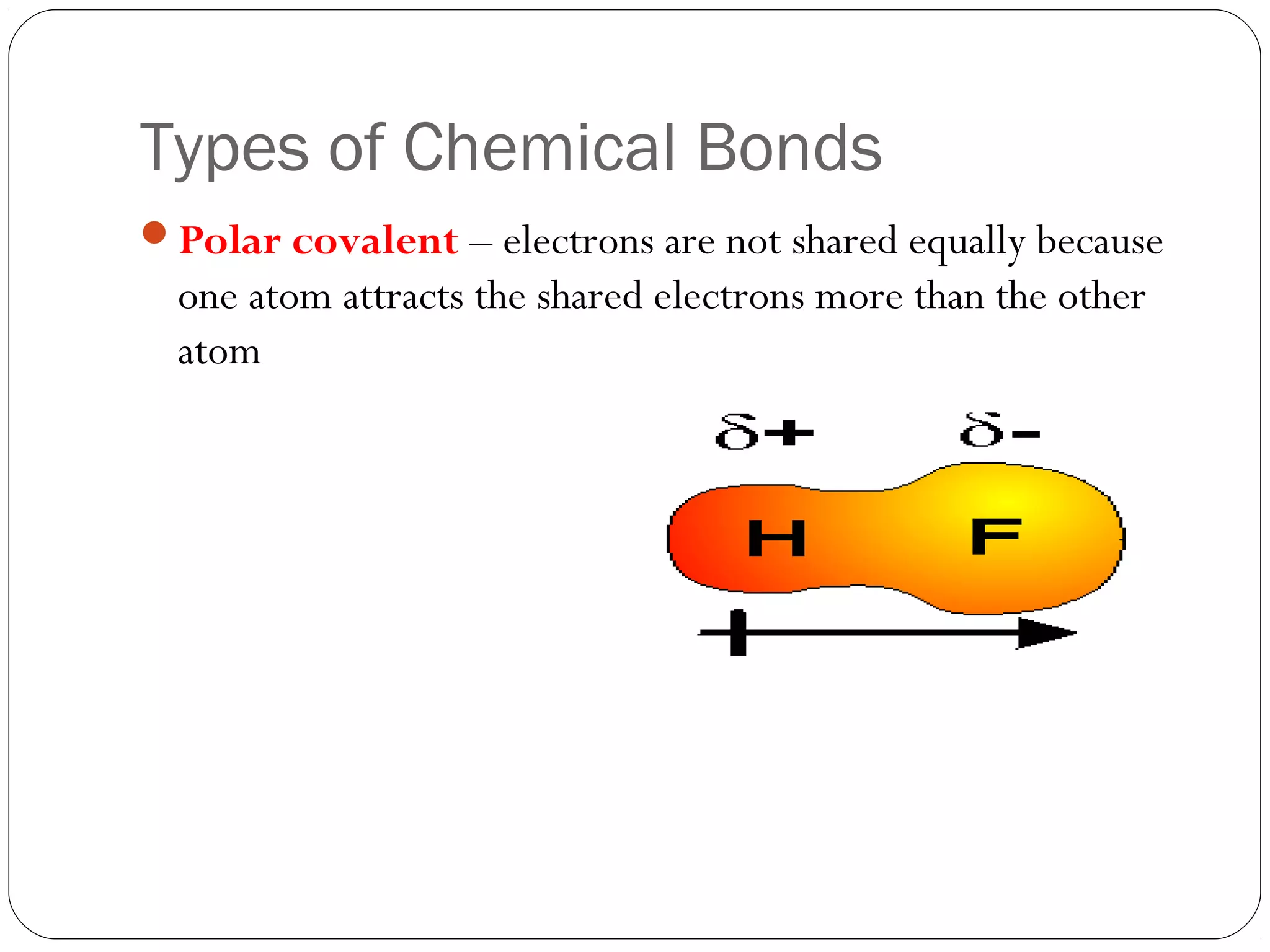
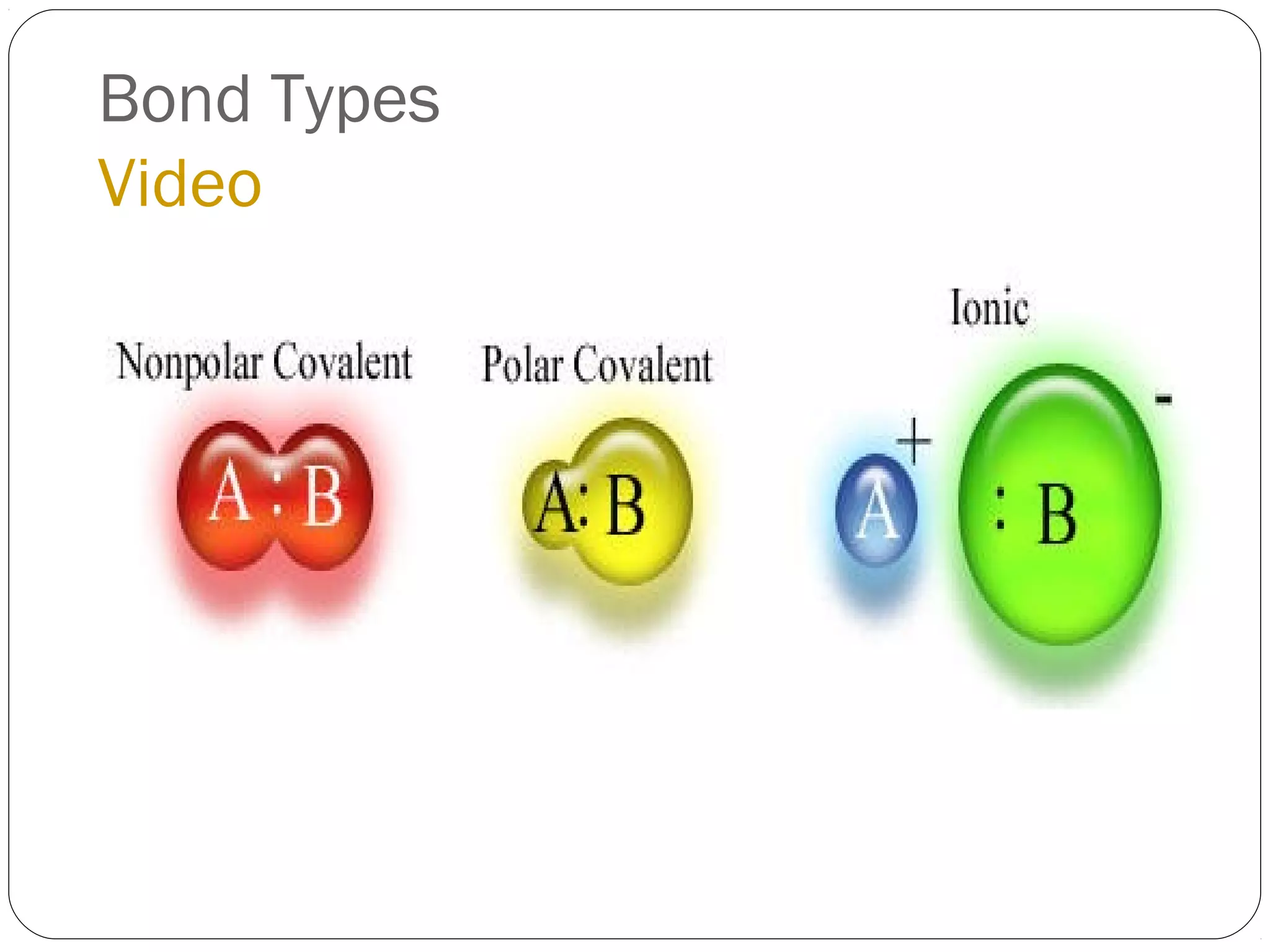
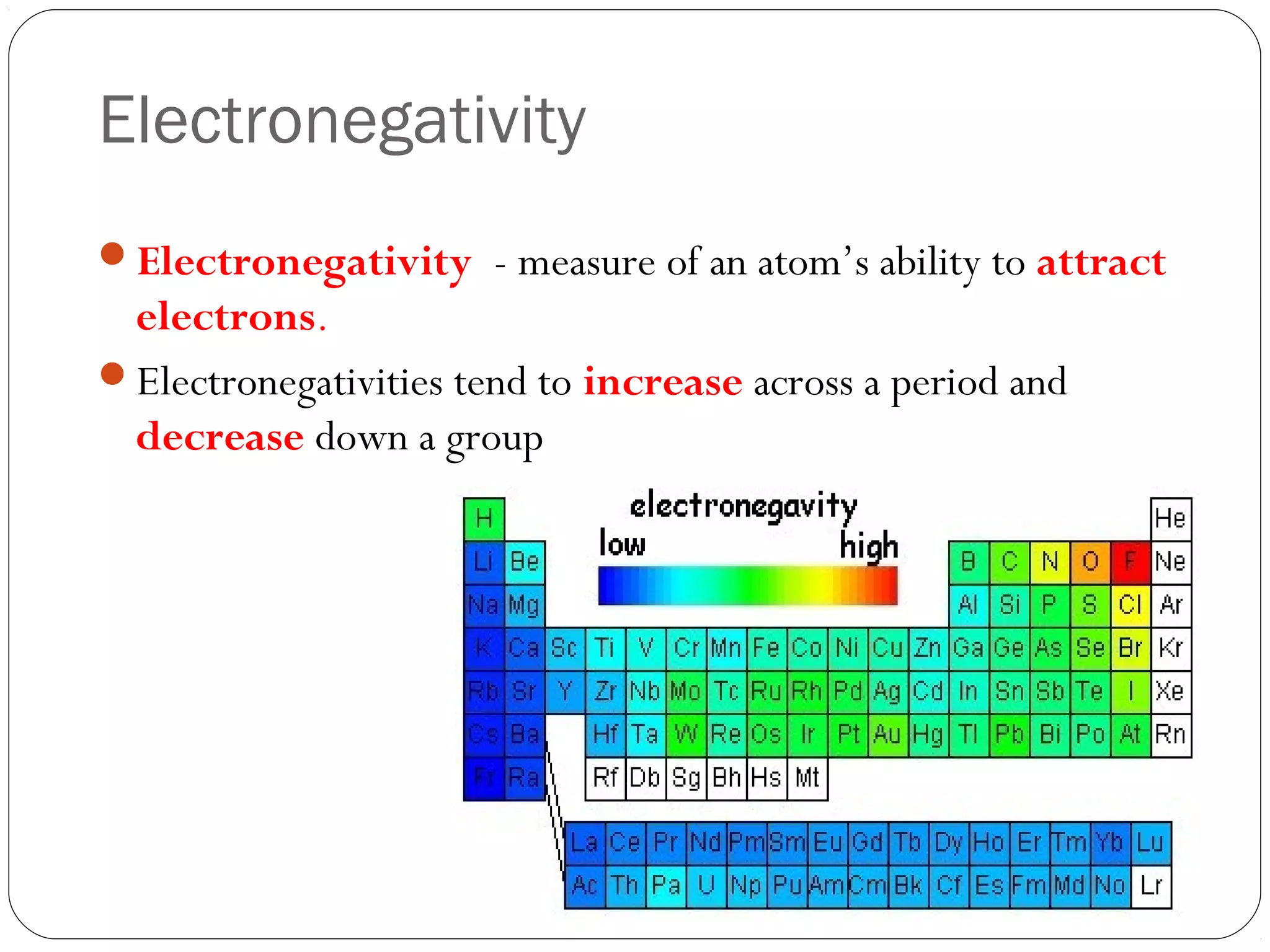
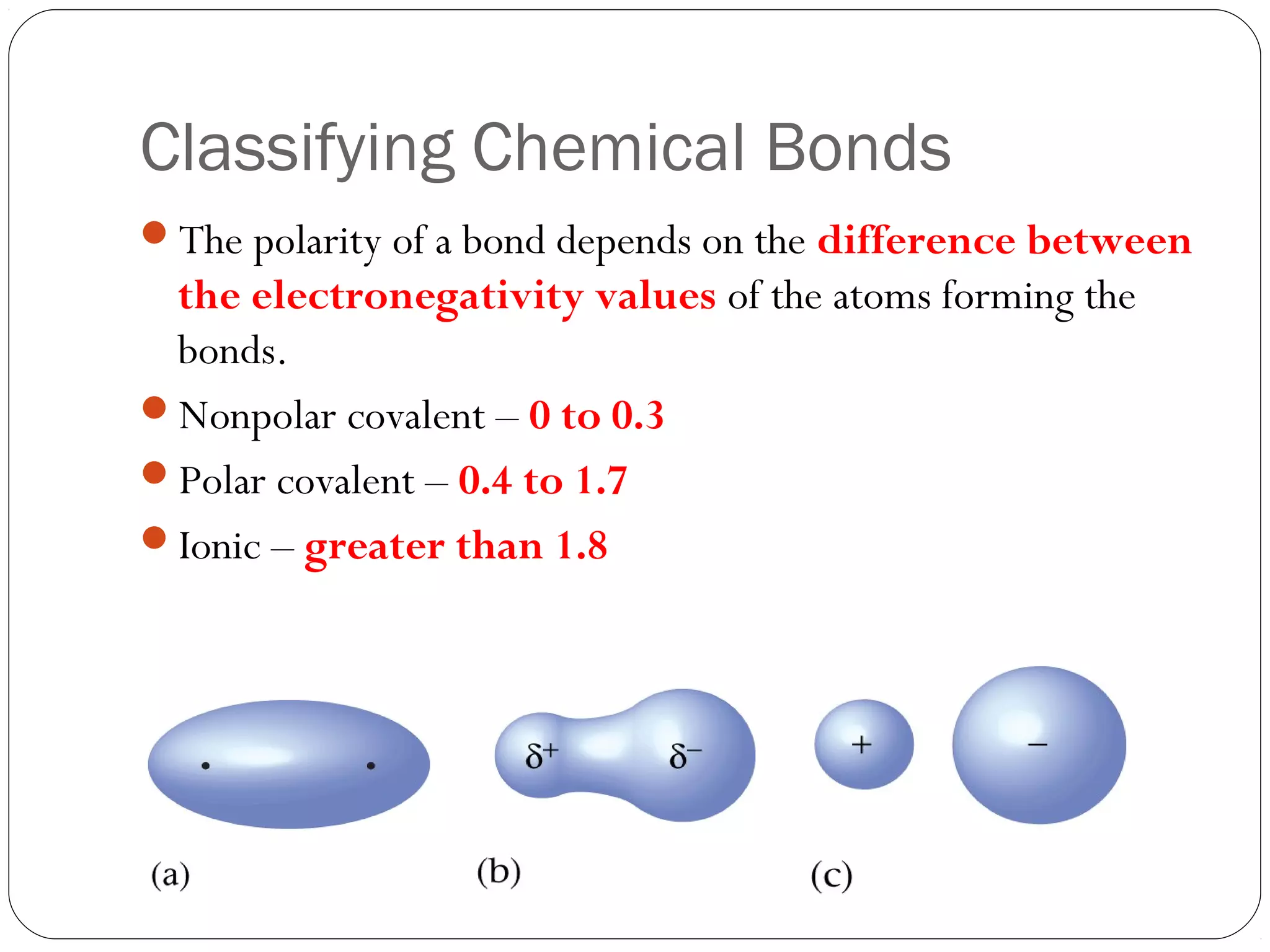
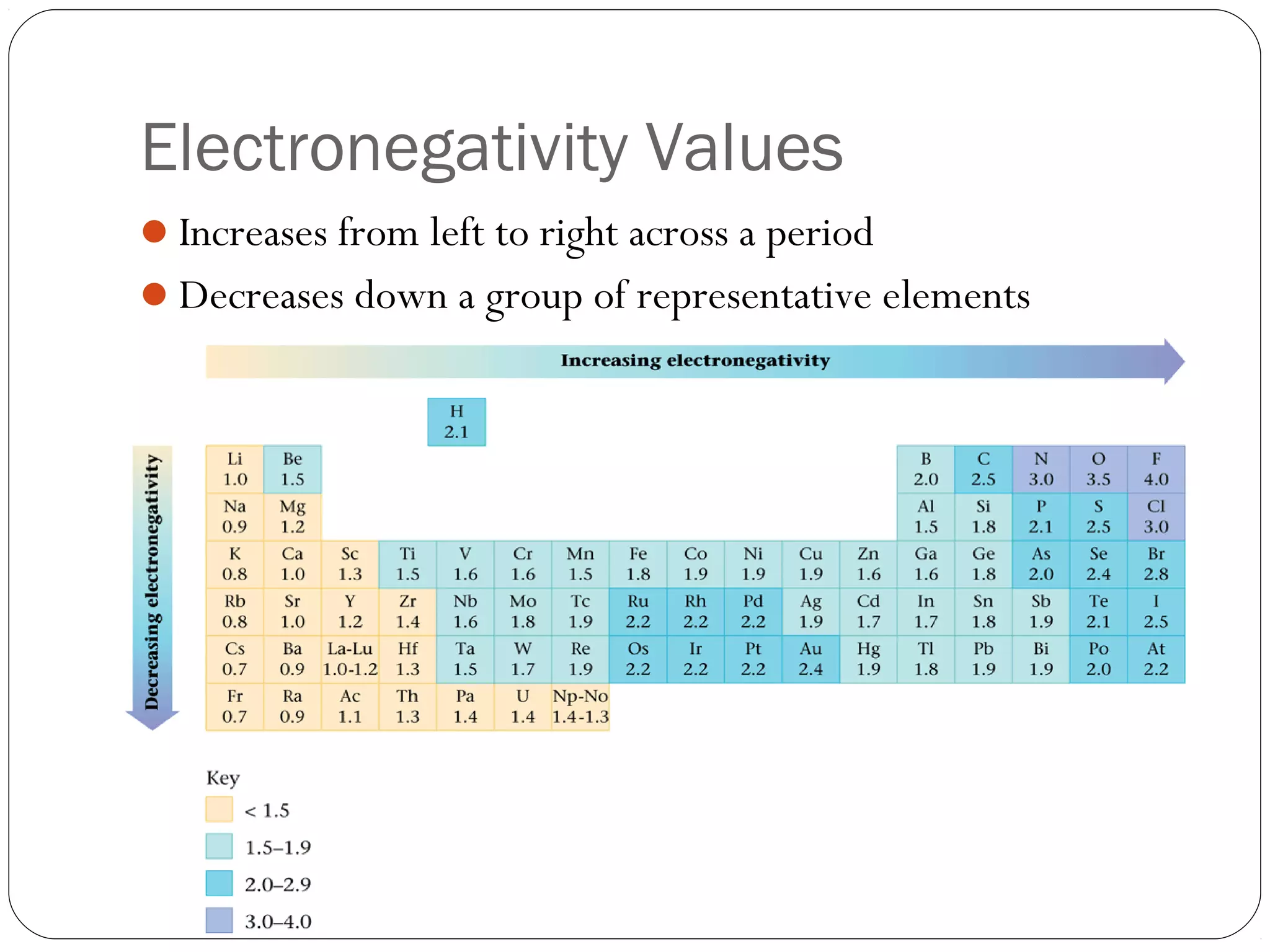
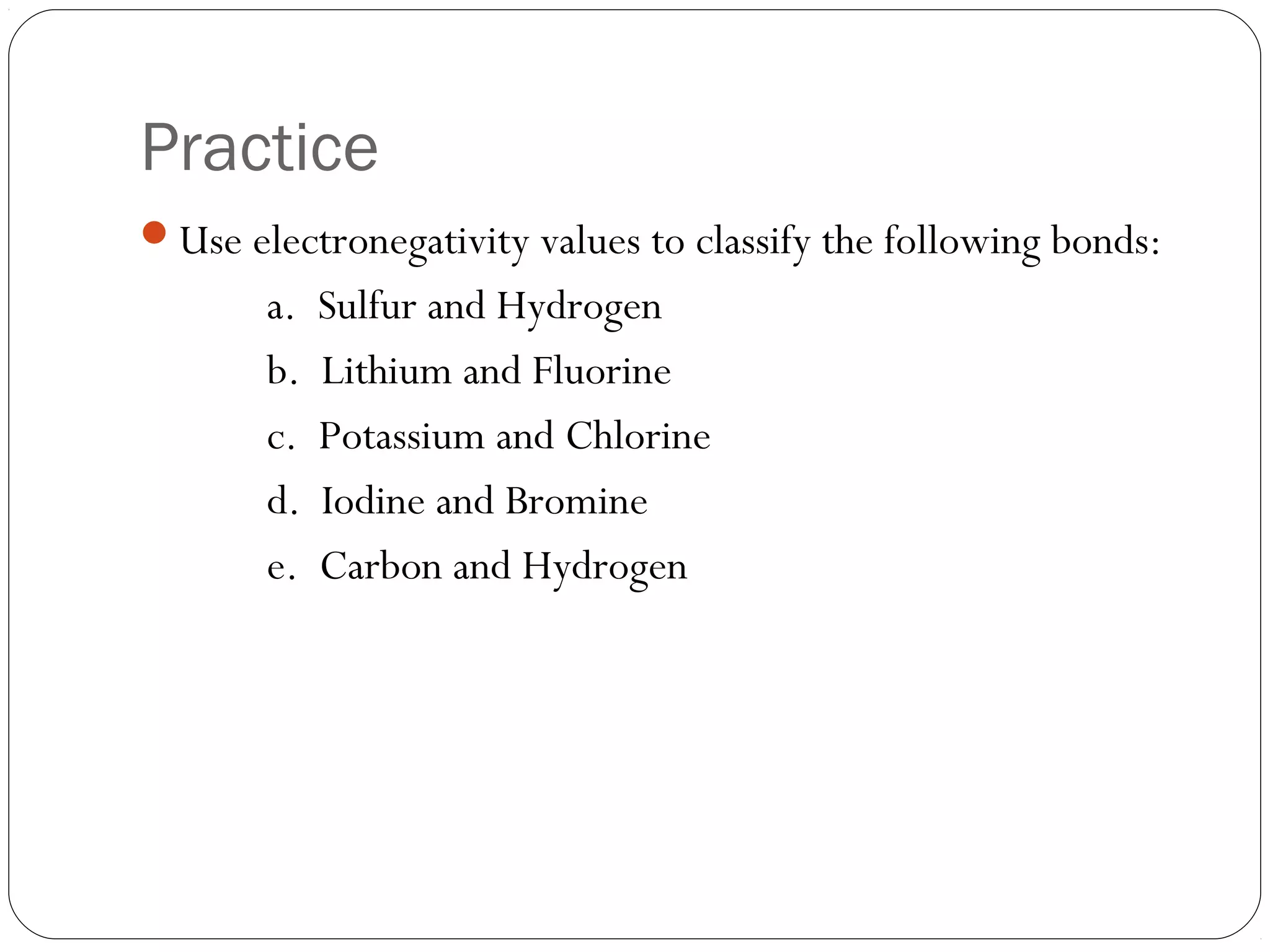
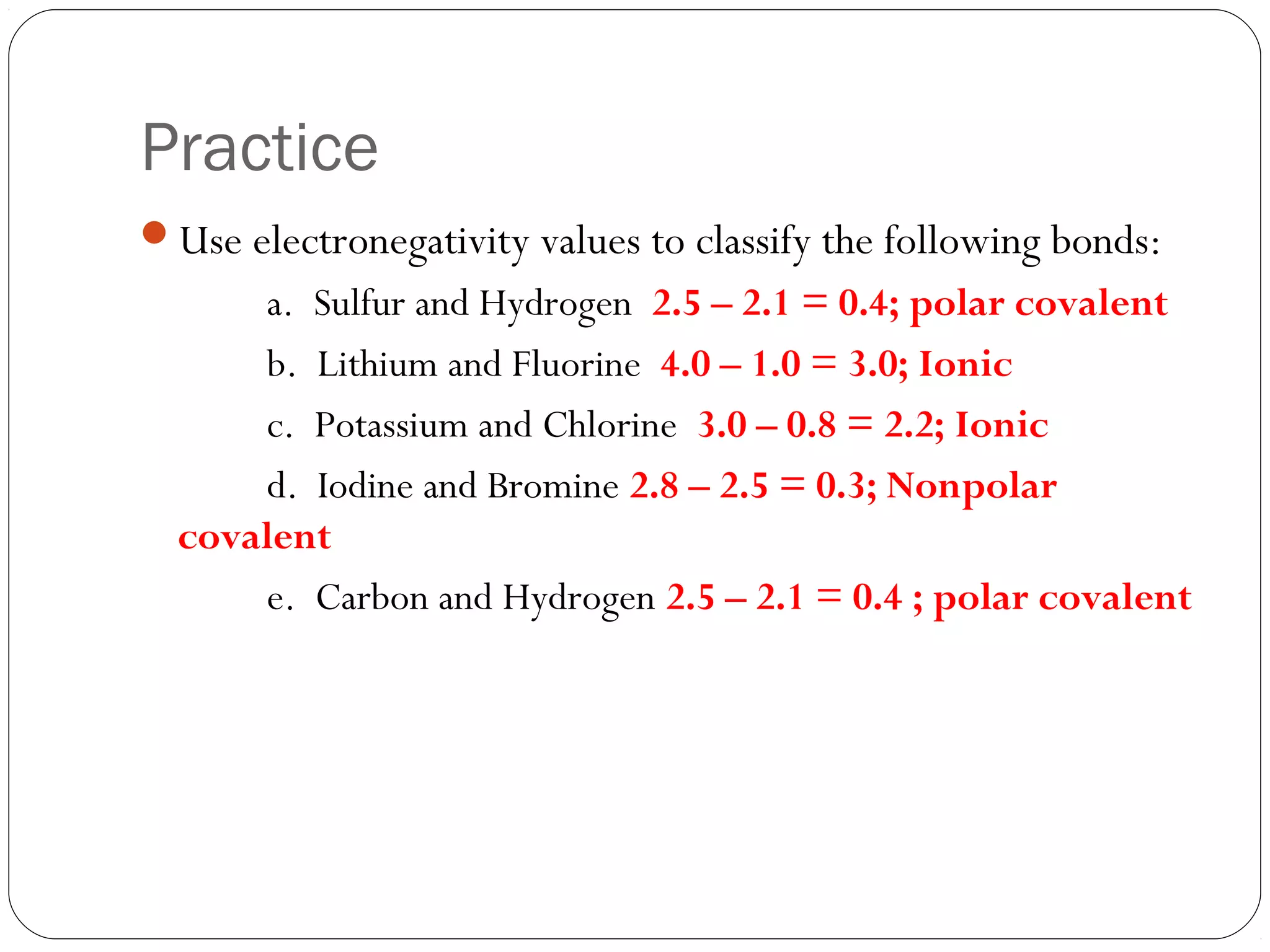
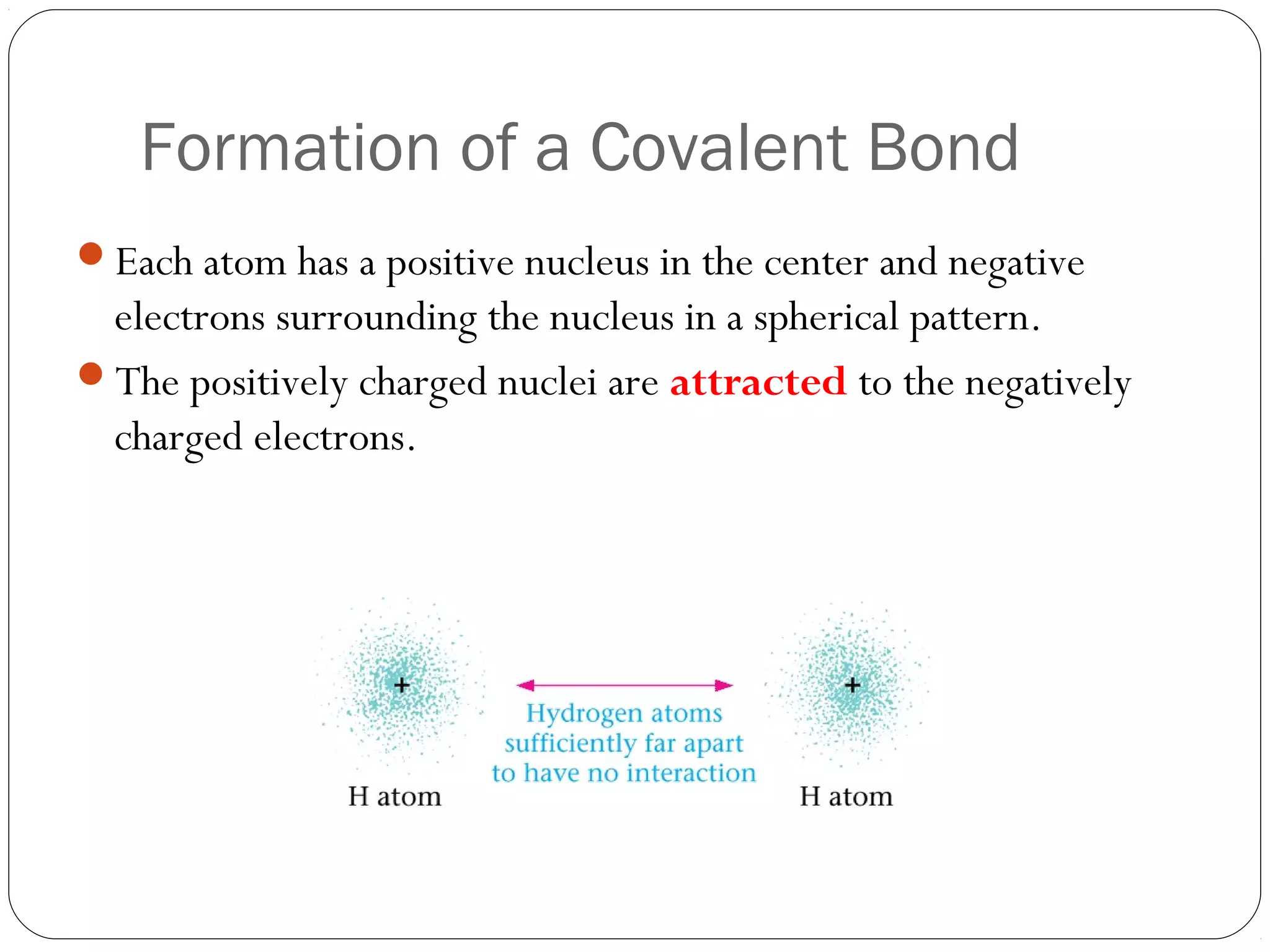
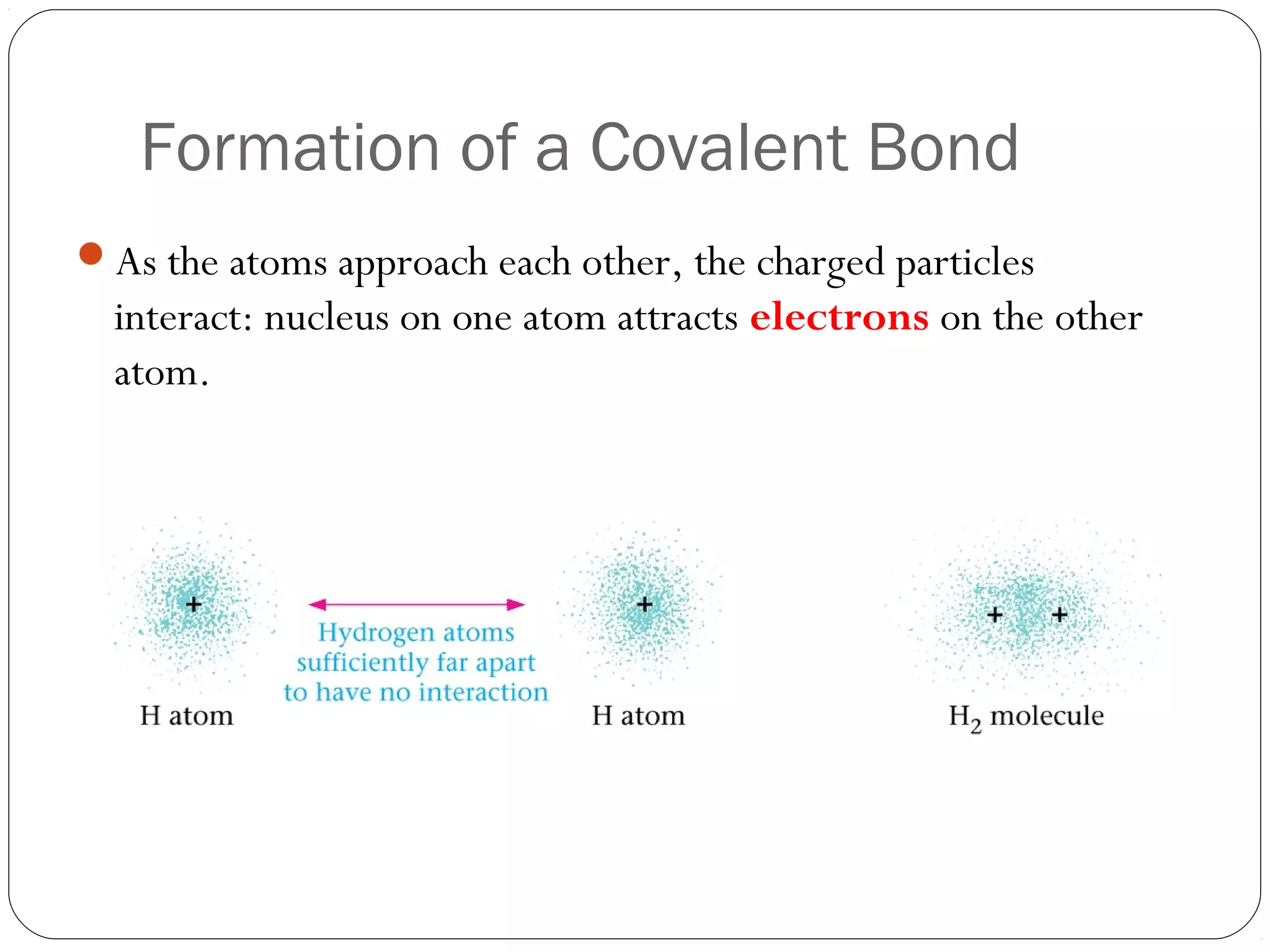
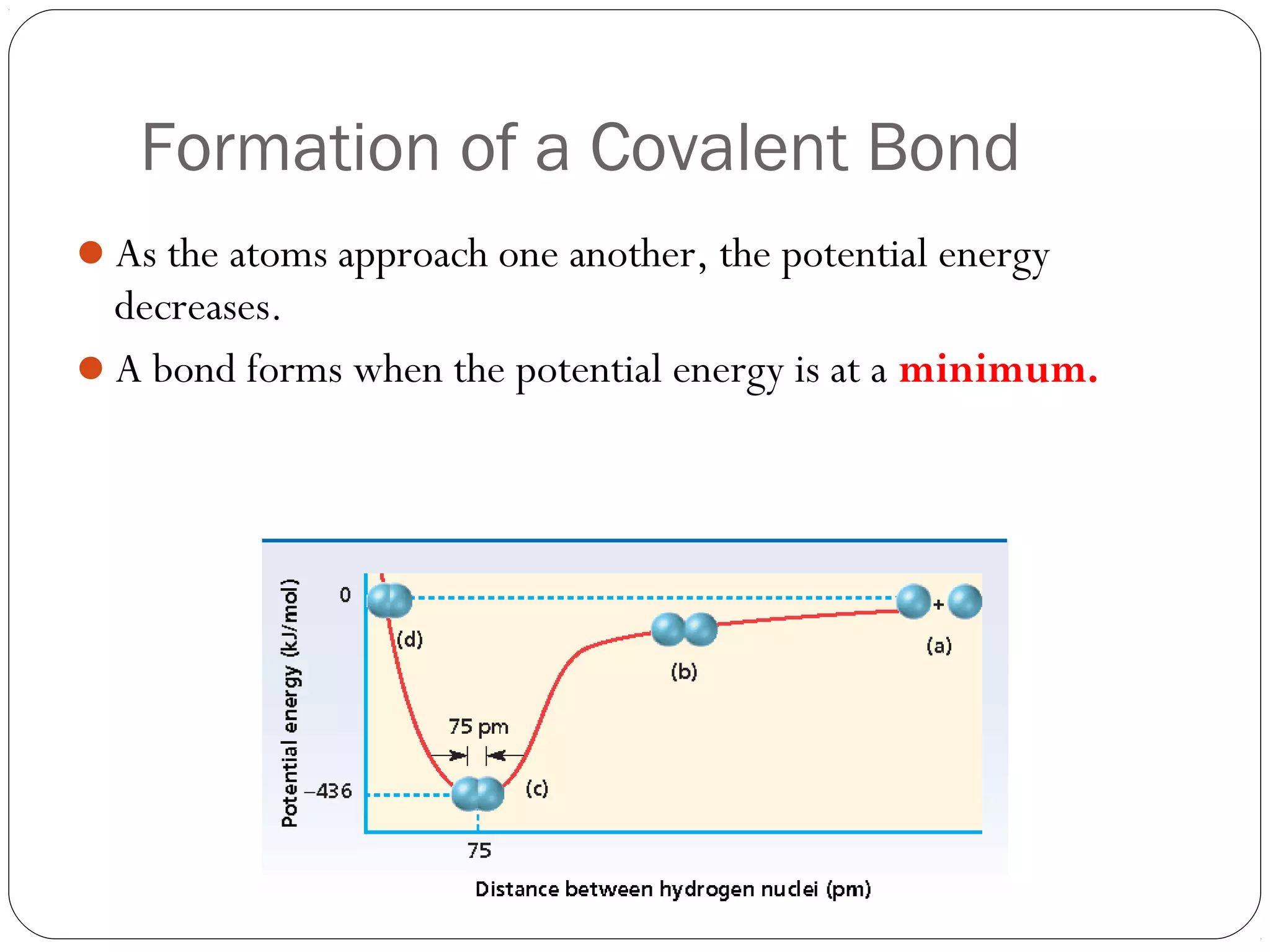
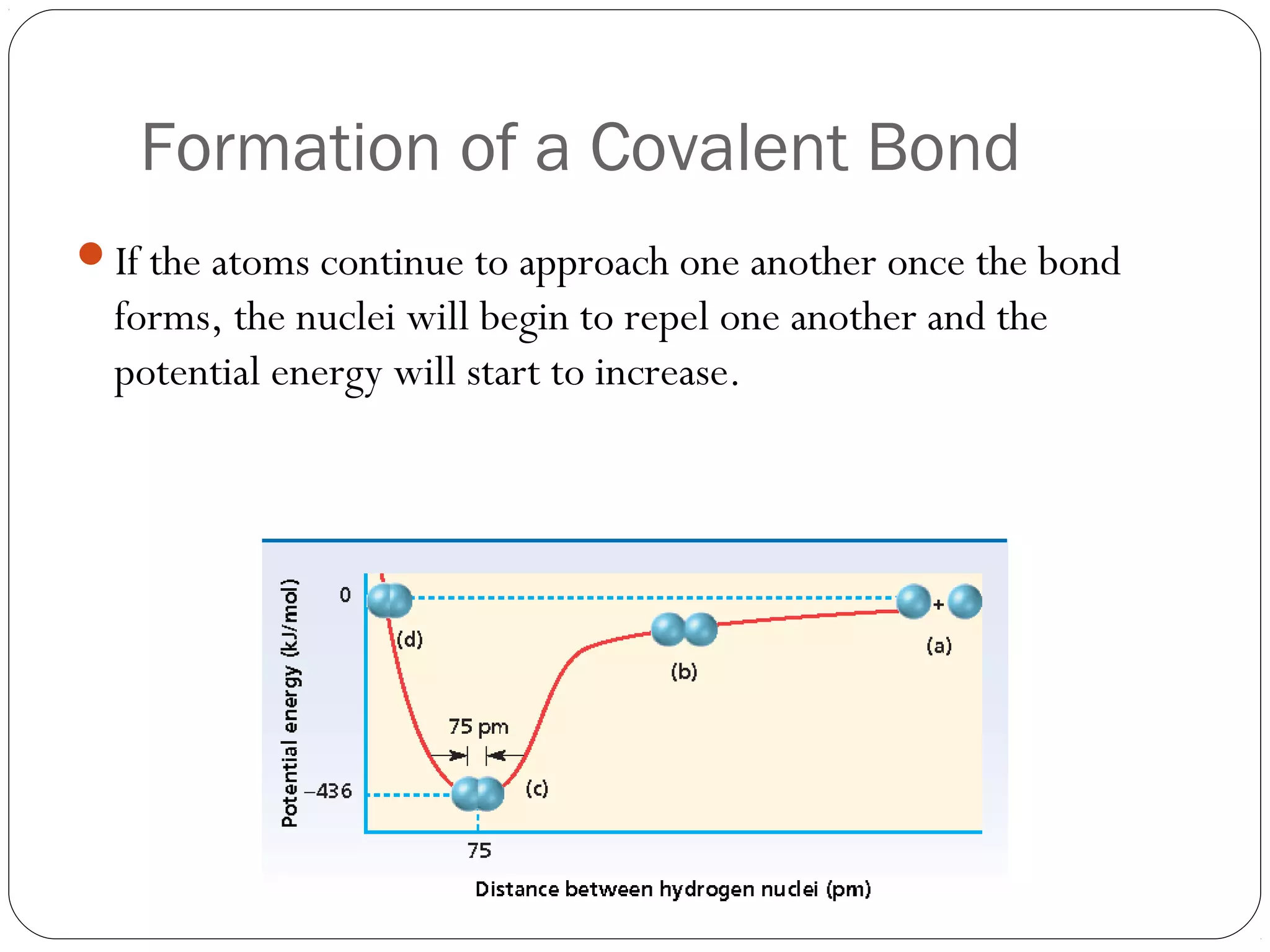
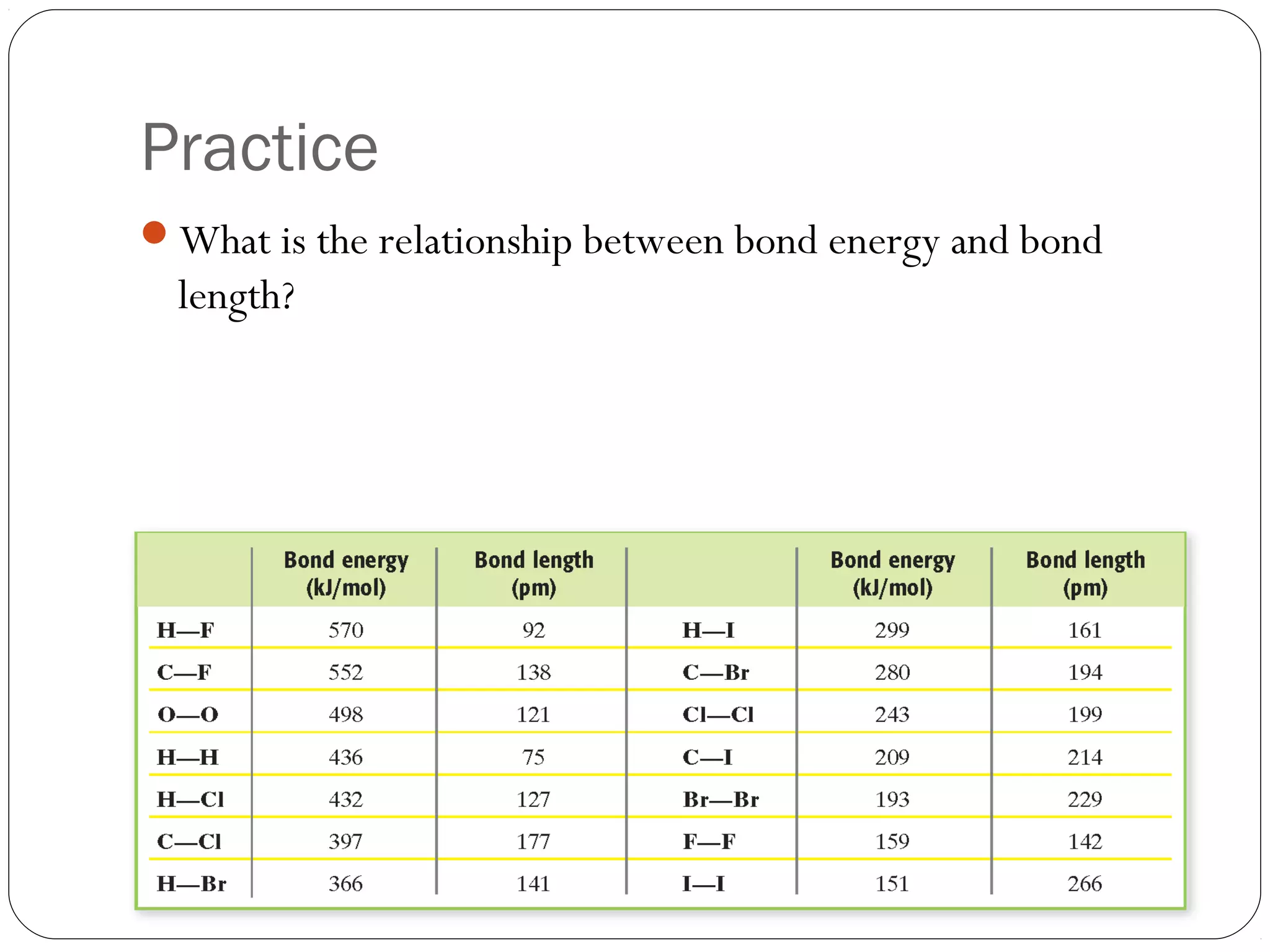
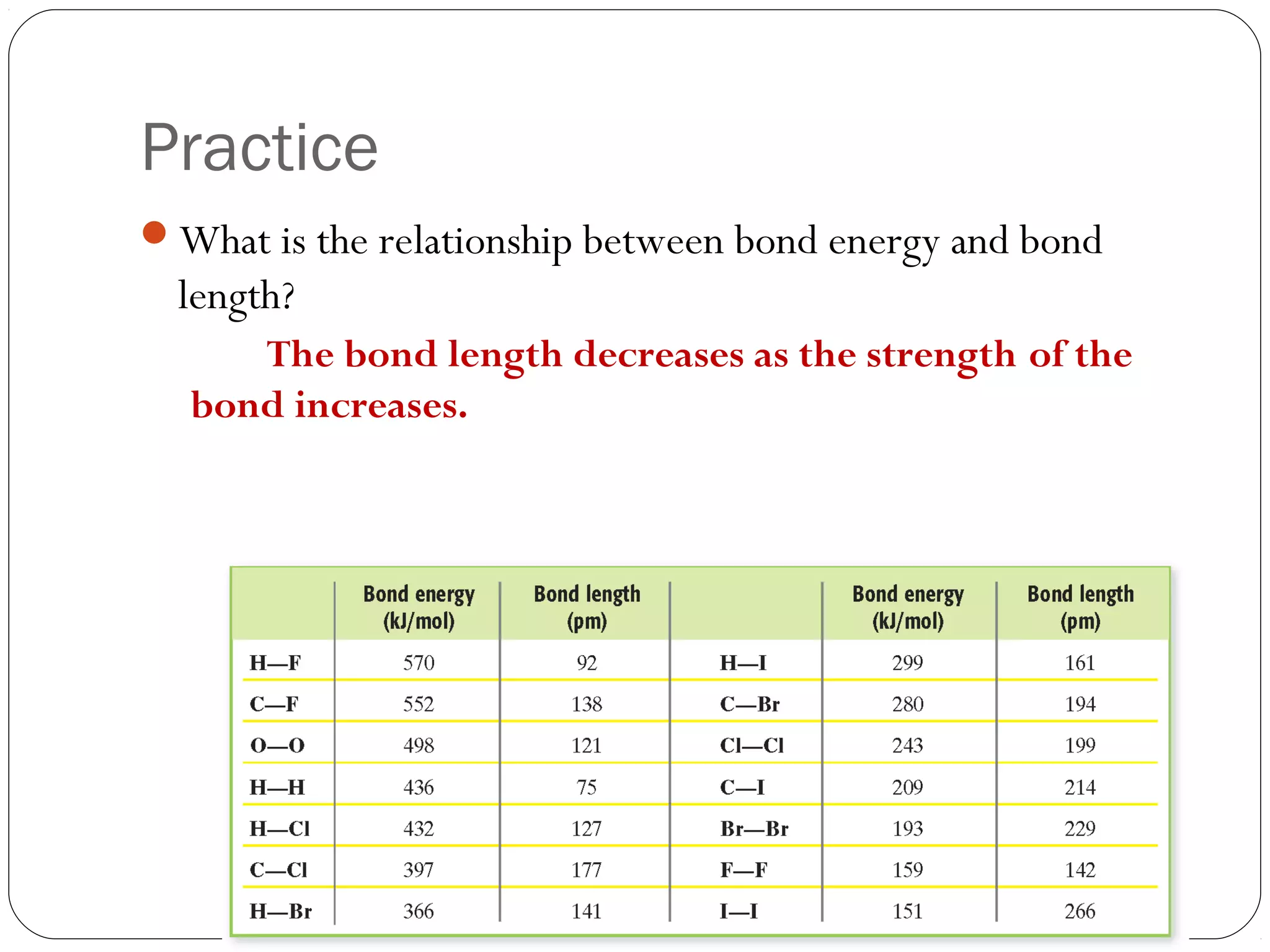
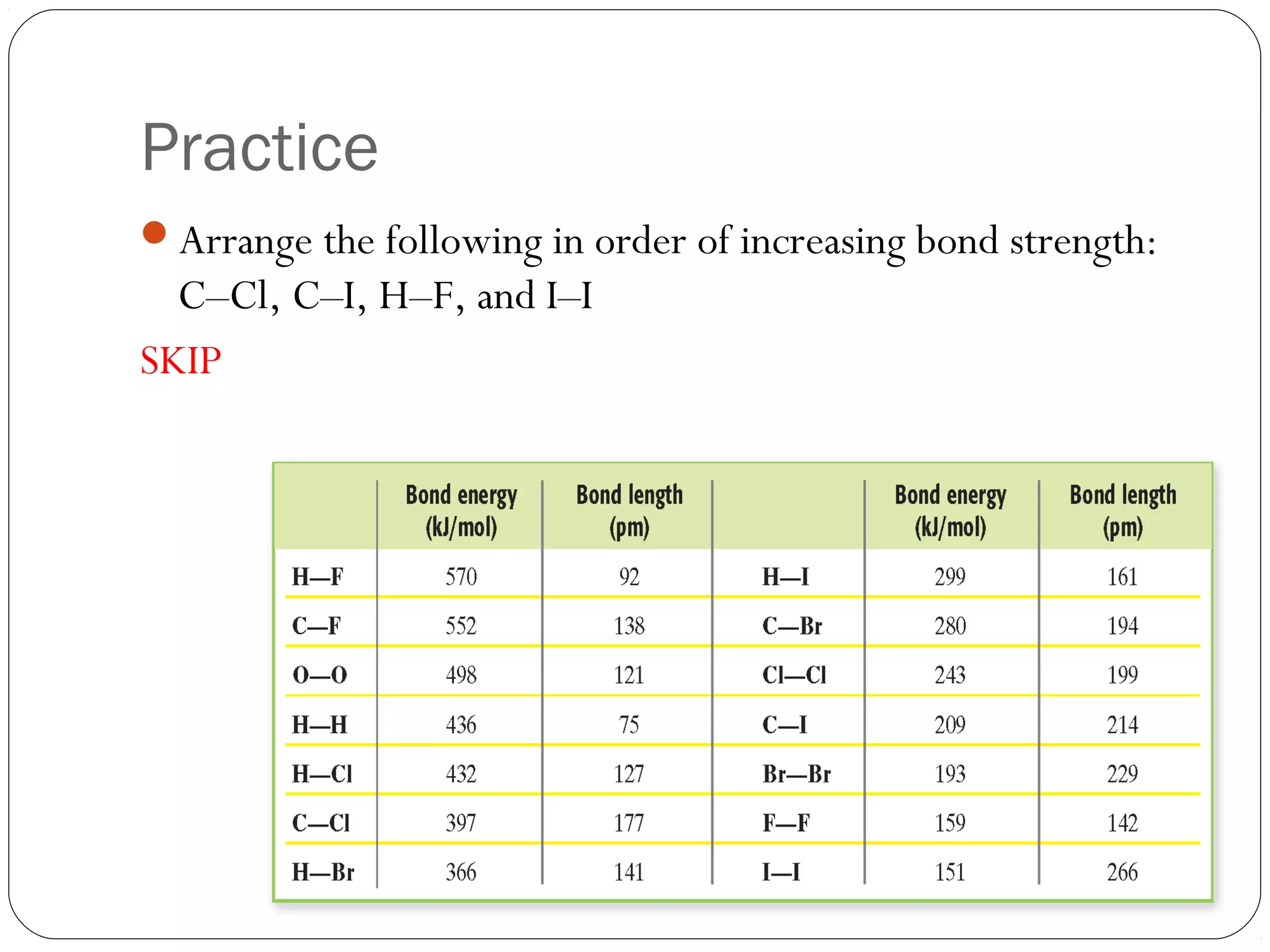
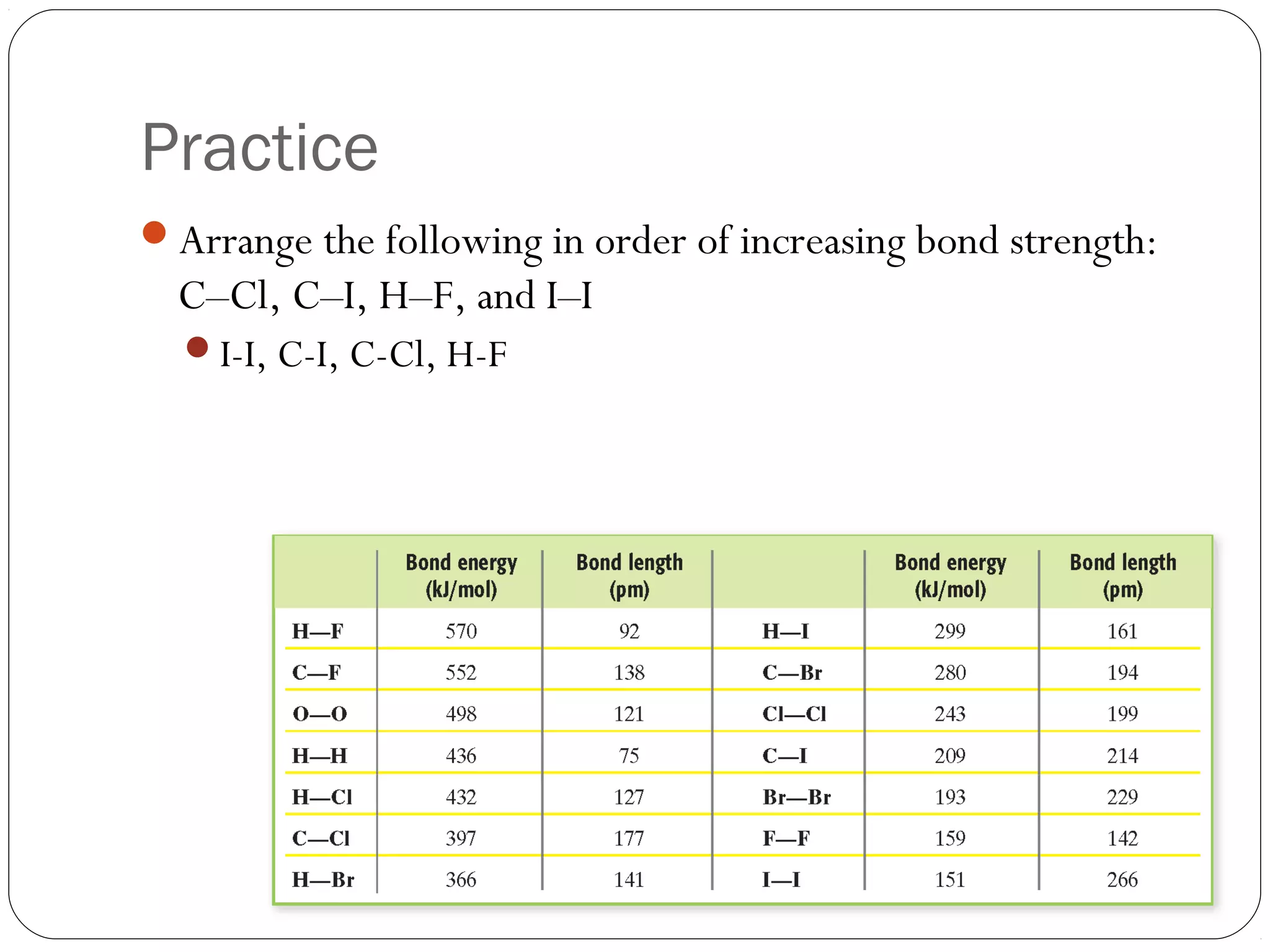
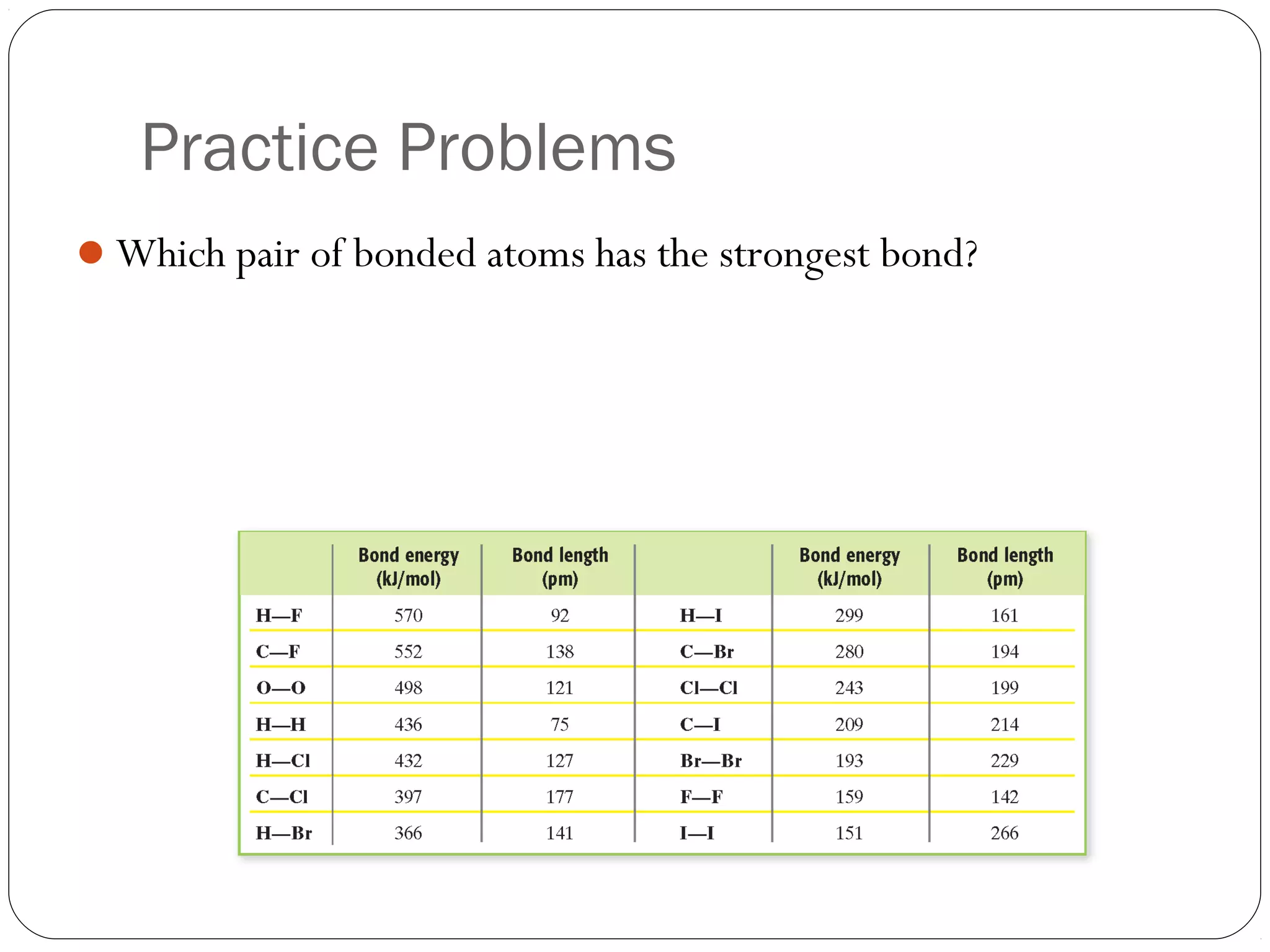
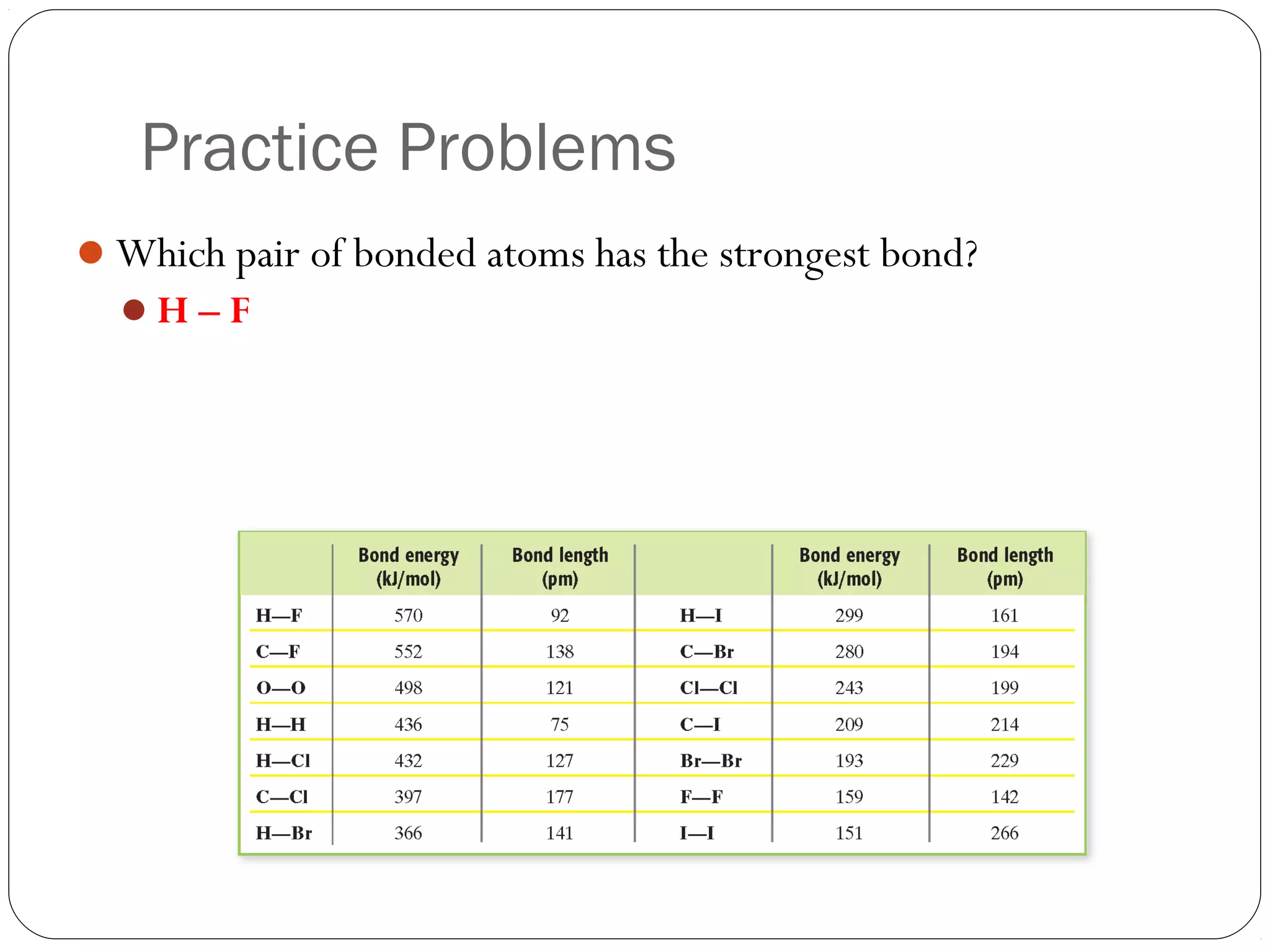
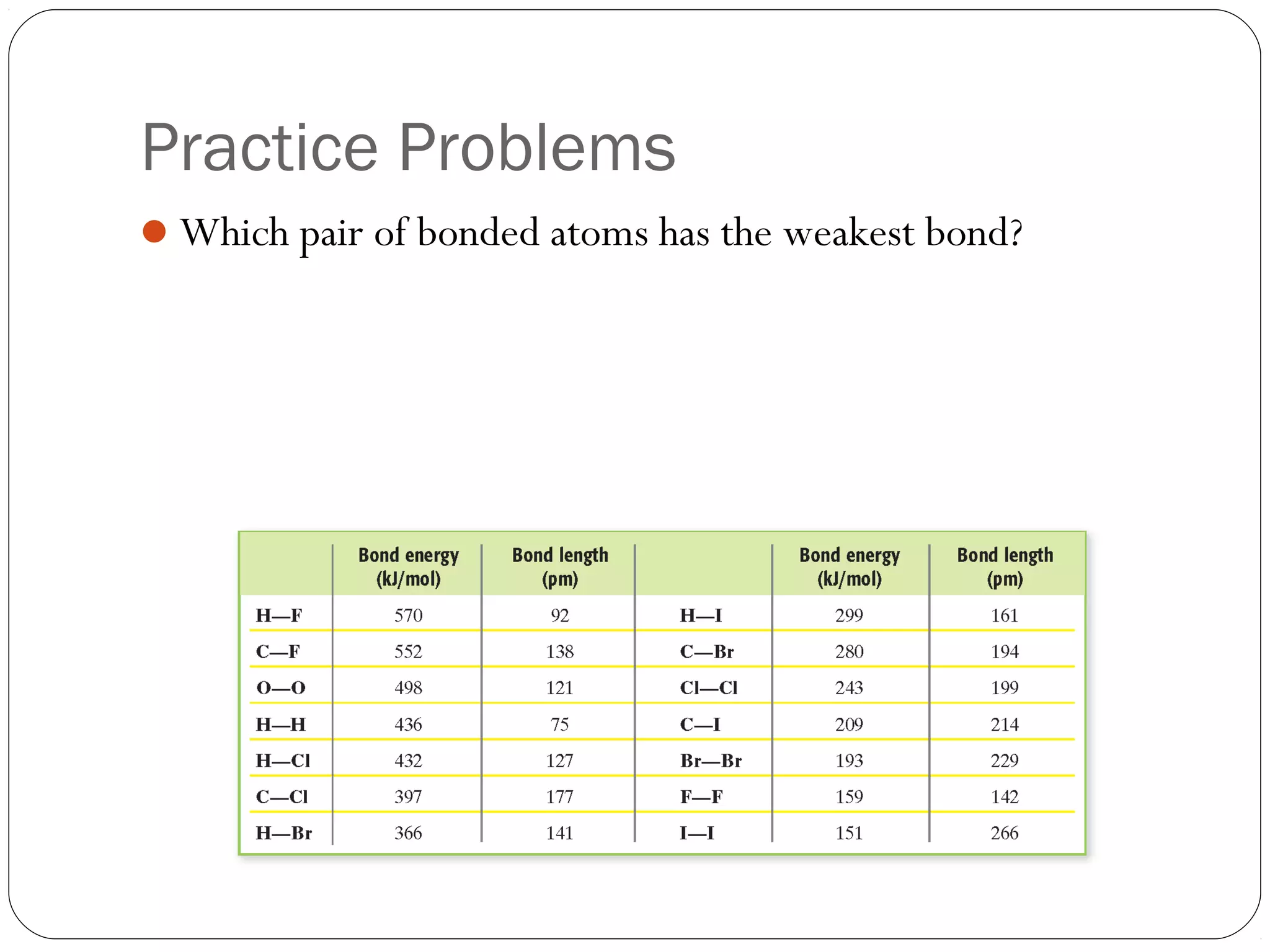
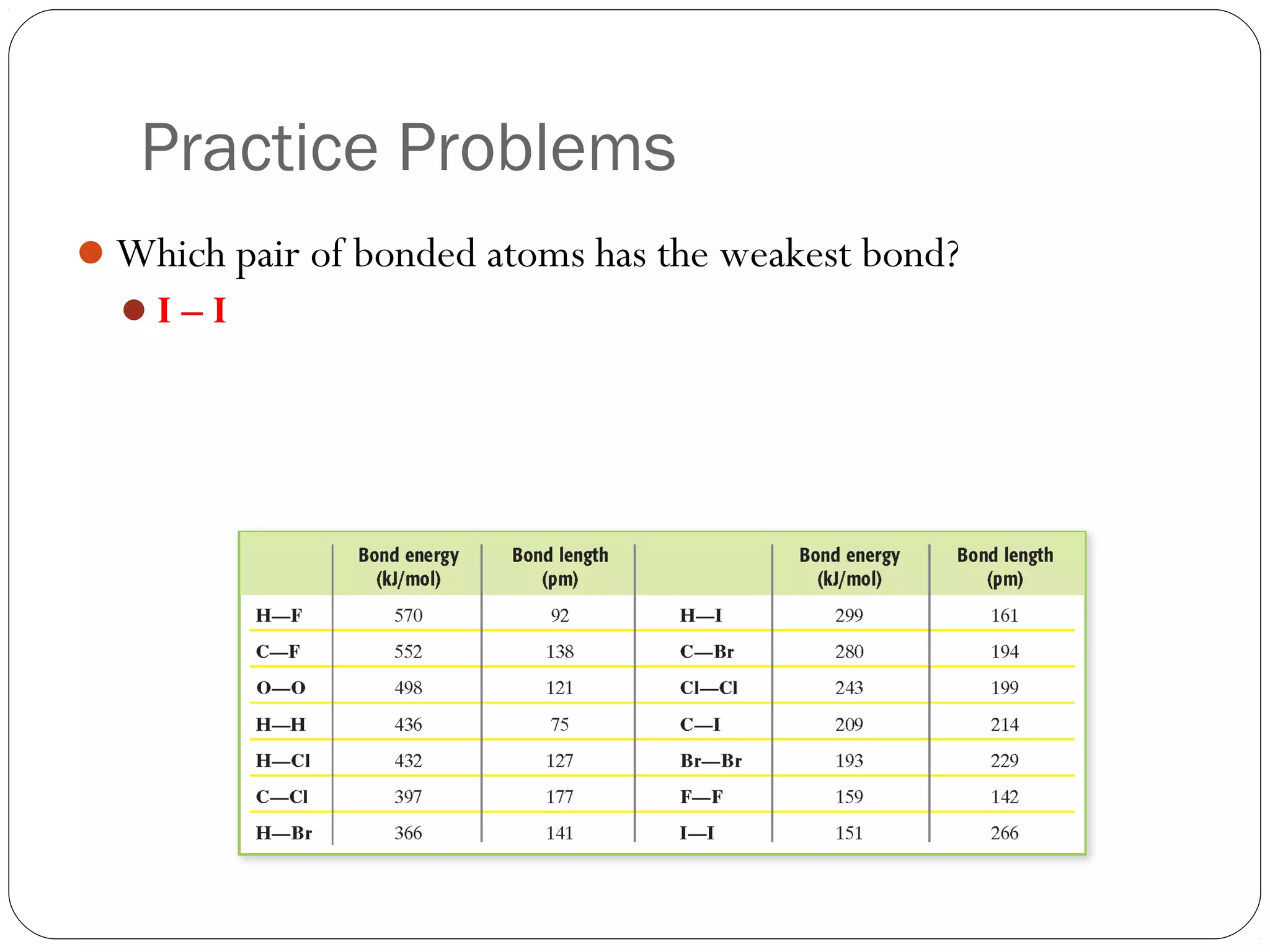
This document discusses covalent bonding and molecular compounds. It defines a chemical bond as a force that holds atoms together, and describes covalent bonding as atoms sharing electrons. As two atoms approach each other to form a bond, their potential energy decreases to a minimum at the bond length. Bond length and bond energy vary between different bonded atoms. The octet rule states atoms want 8 electrons in their valence shell. Practice problems classify bonds and identify valence electrons.