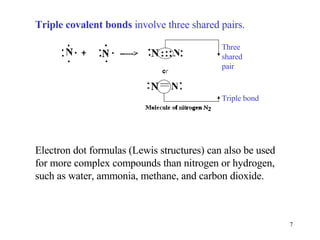
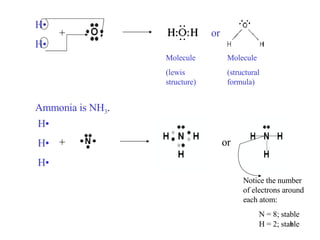
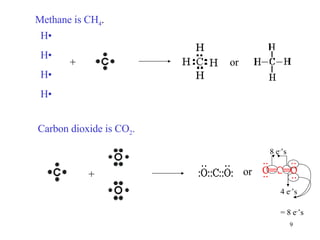
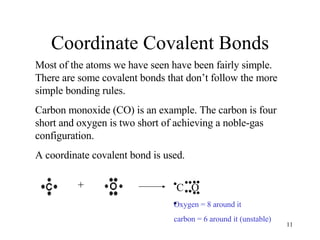
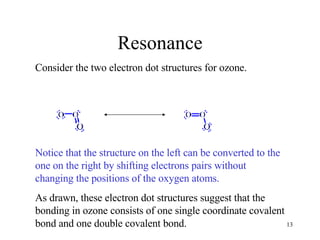
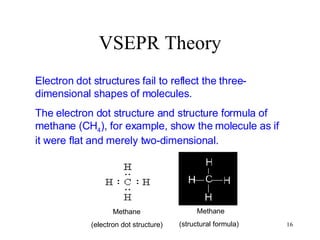
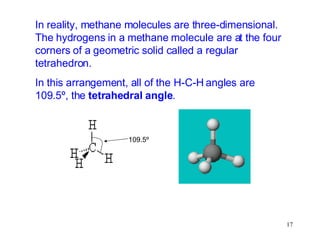
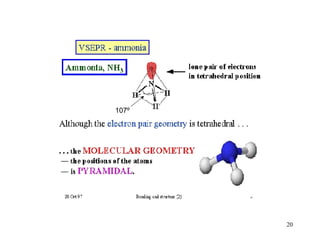
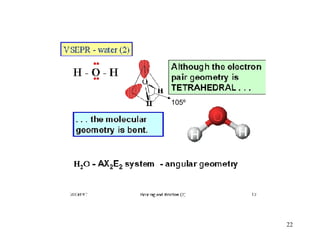
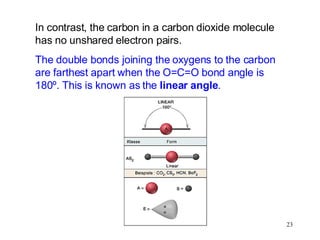
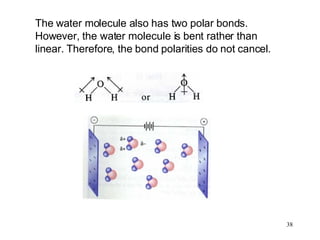
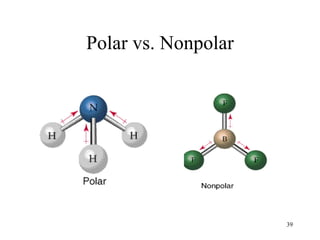
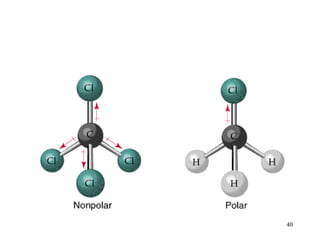
The document discusses different types of covalent bonds:
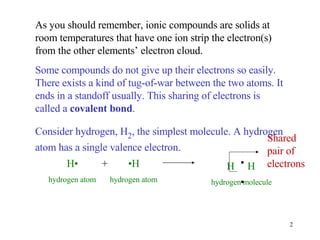
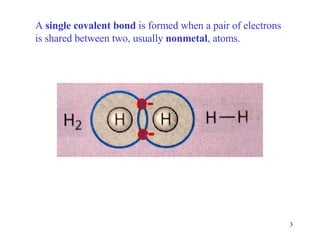
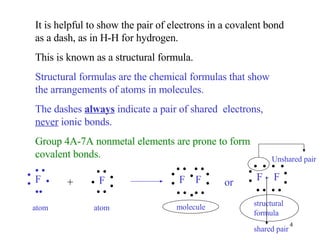
- Single covalent bonds involve one shared pair of electrons between two nonmetal atoms.
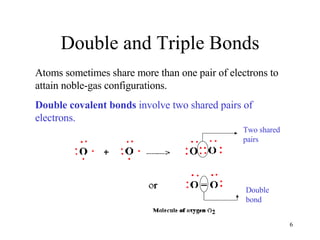
- Double and triple covalent bonds share two or three pairs of electrons respectively.
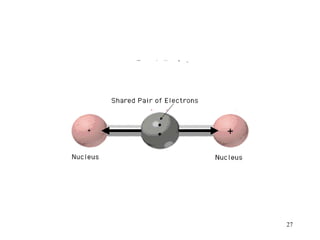
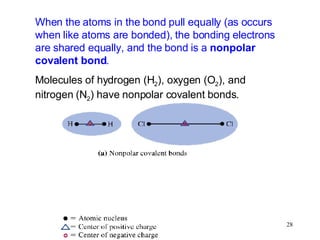
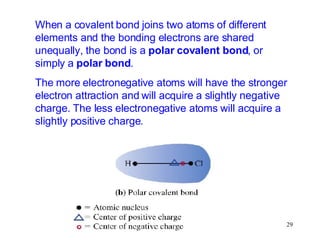
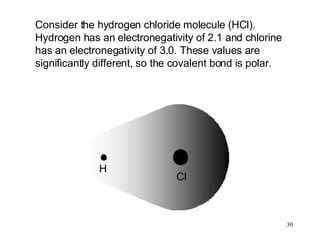
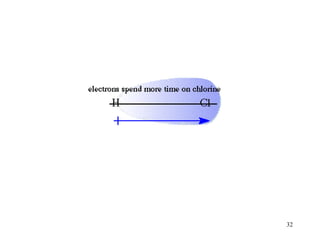
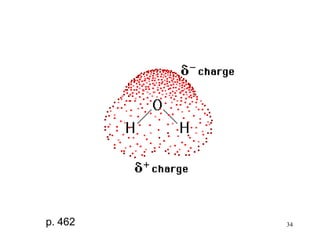
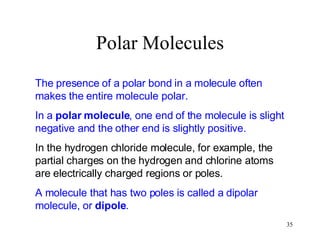
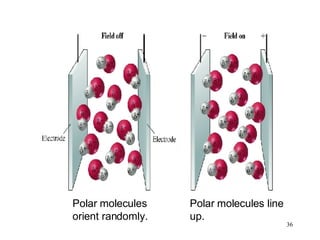
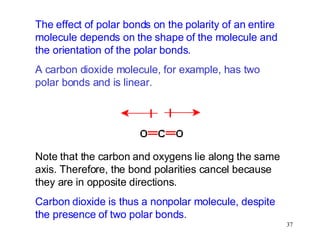
- Polar covalent bonds occur when electrons are shared unequally between atoms of different electronegativity, giving the atoms partial positive and negative charges. Polar molecules have regions of positive and negative charge.