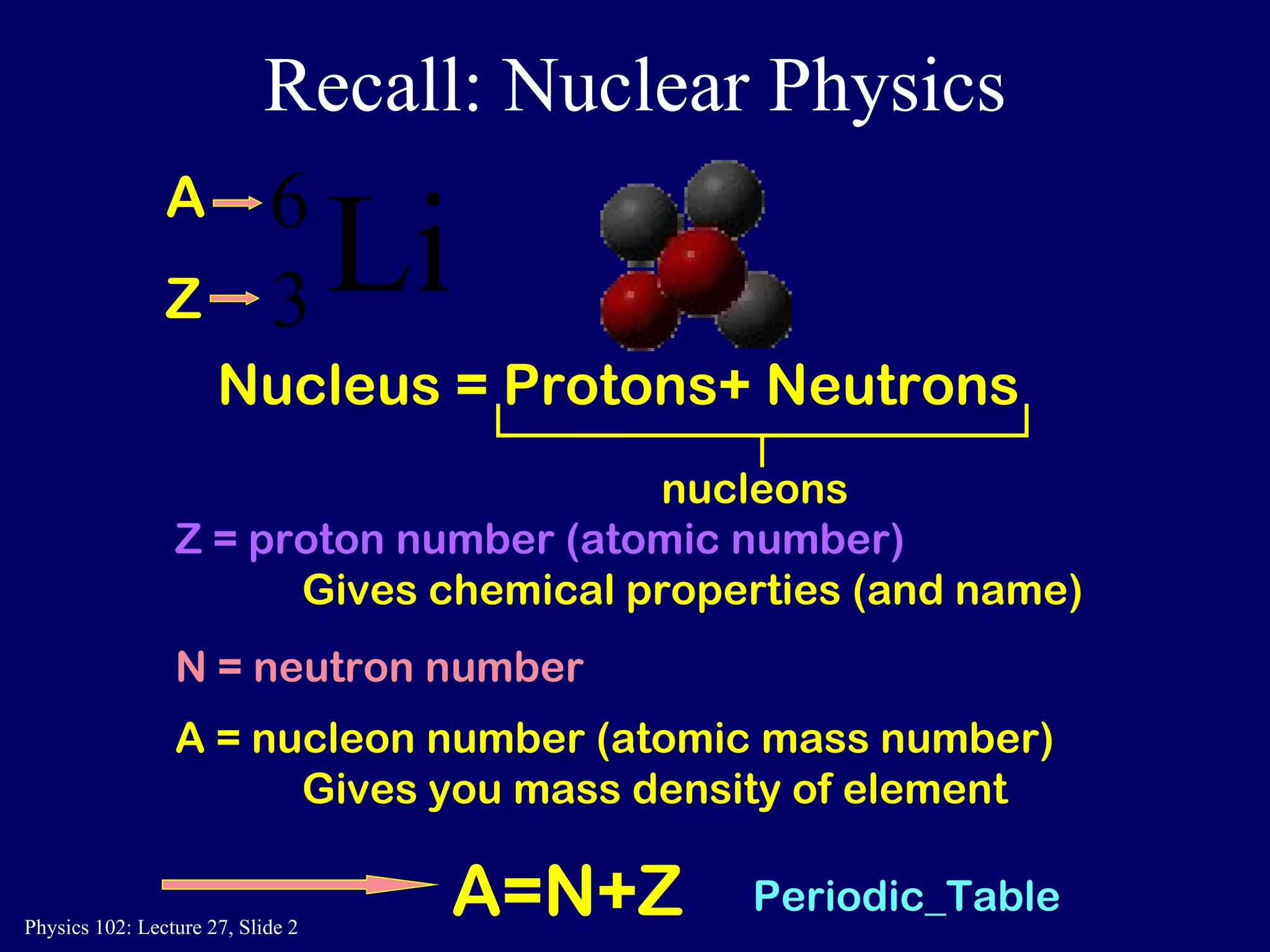
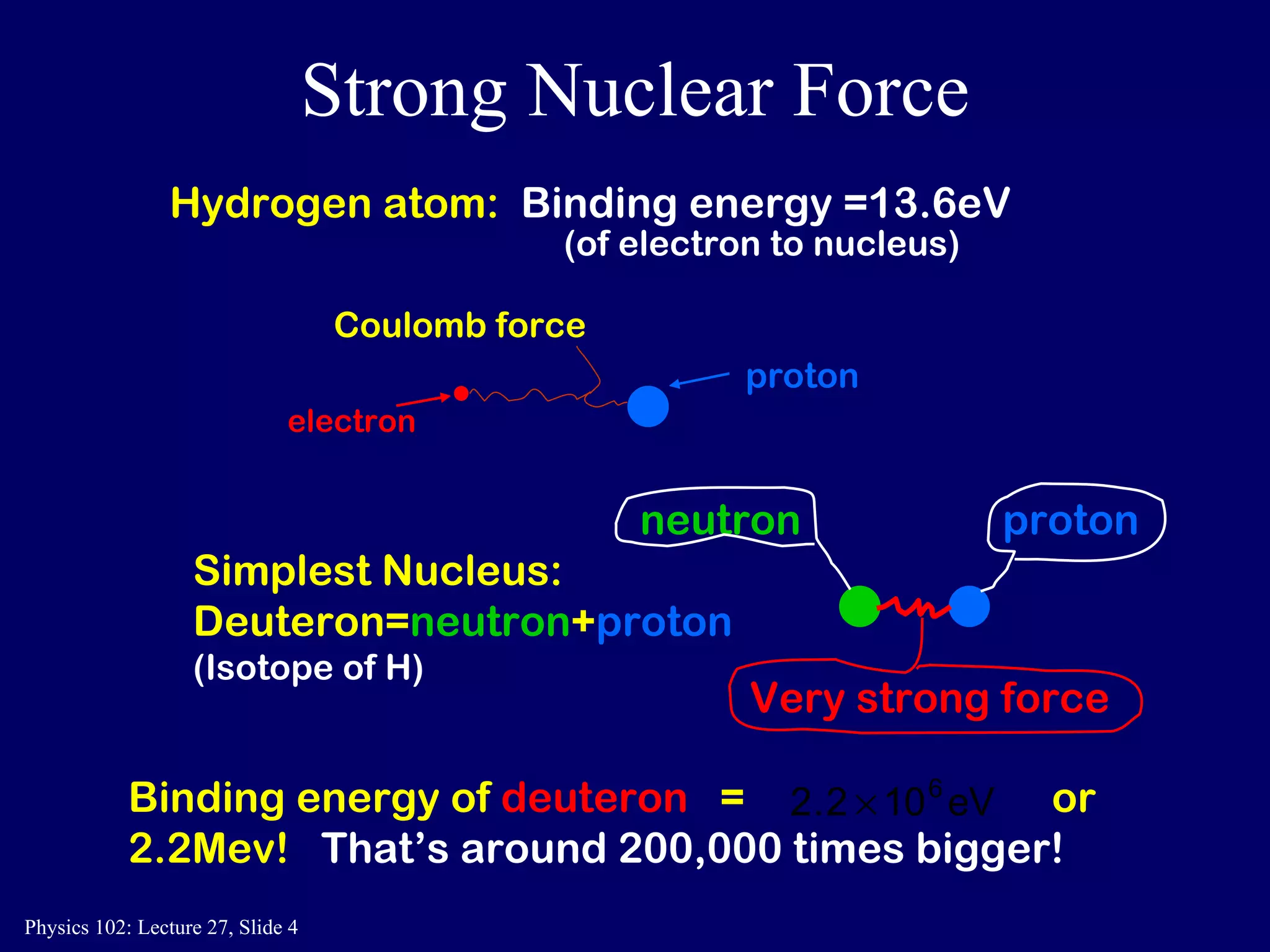
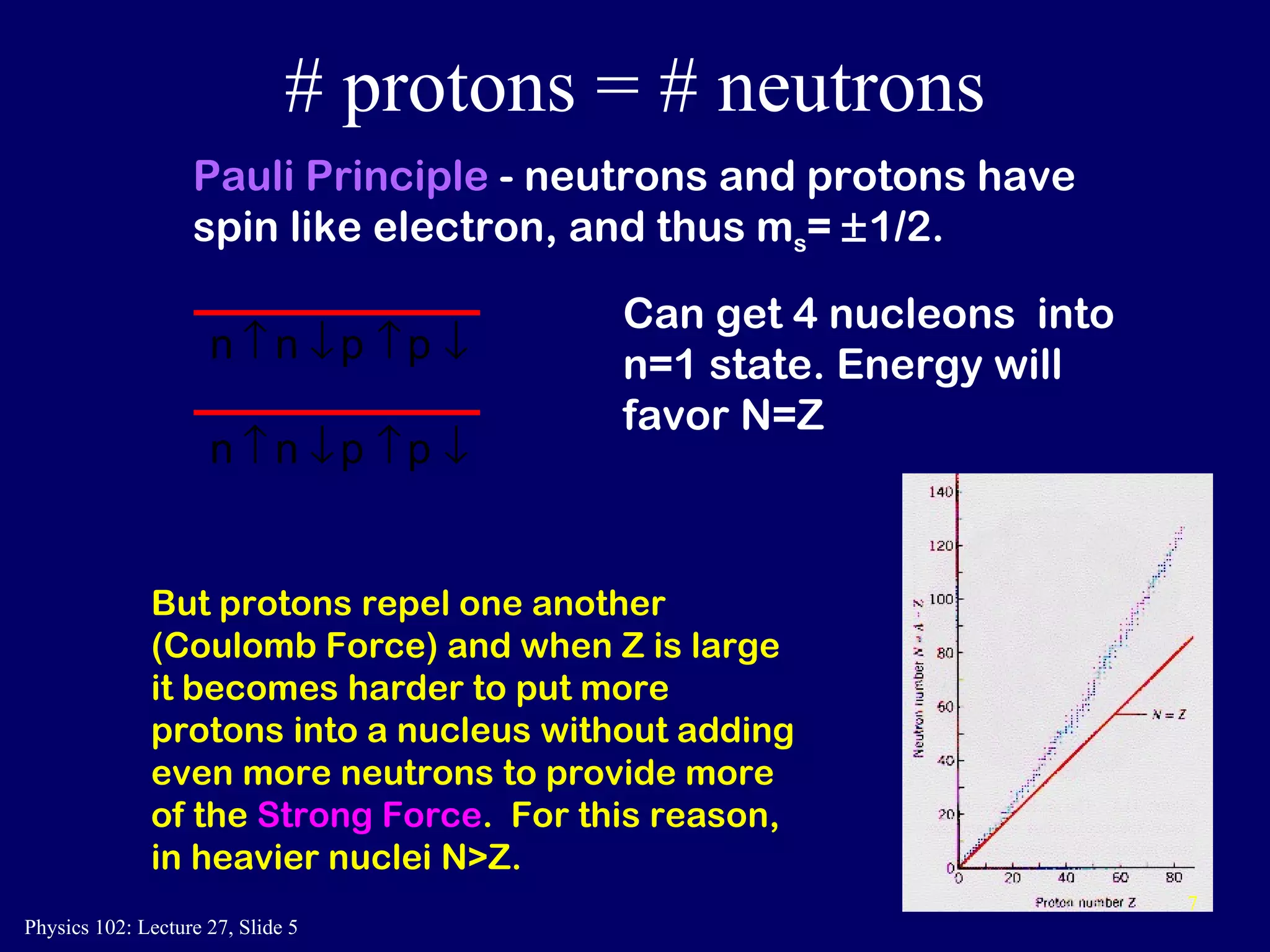
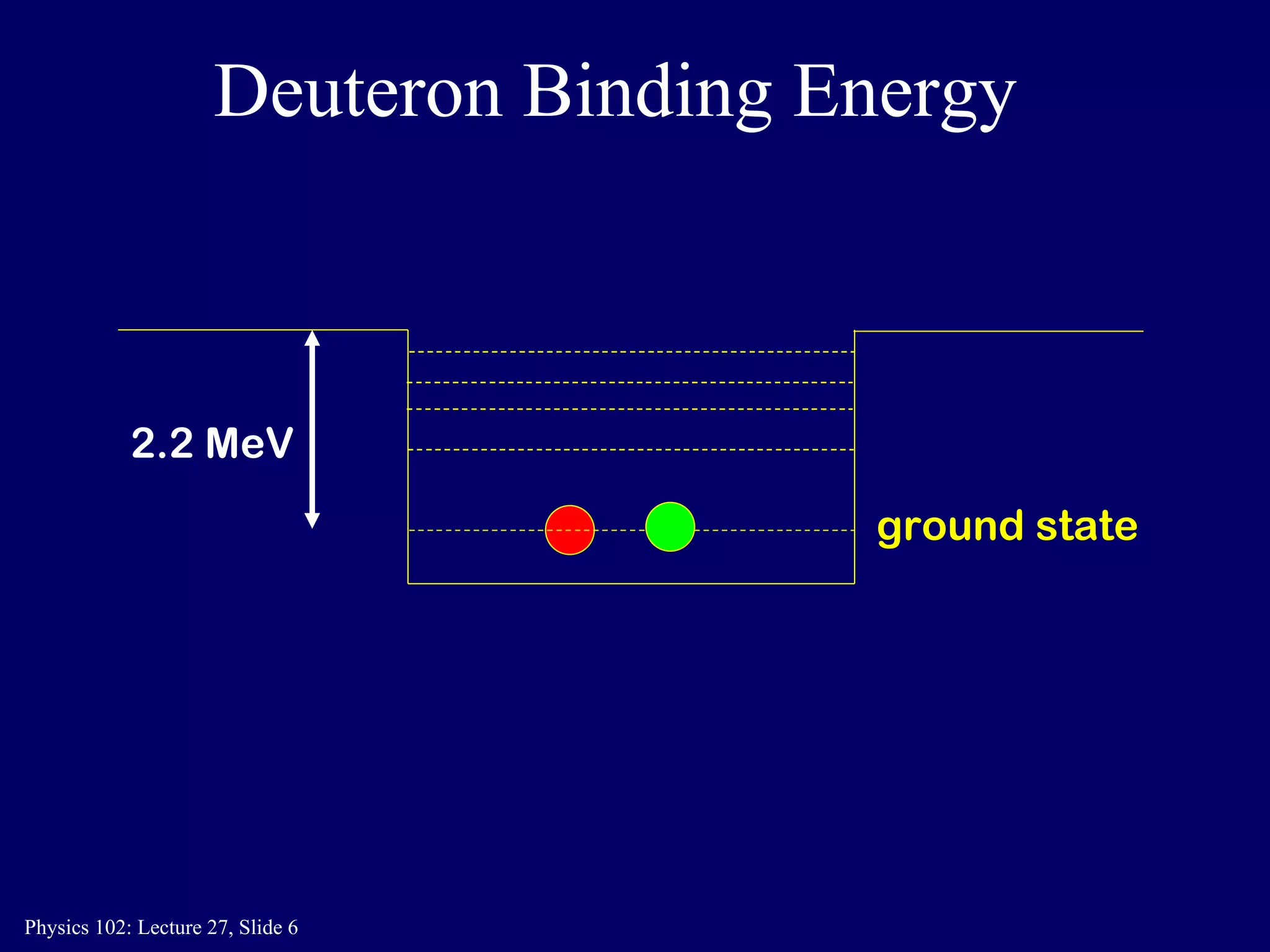
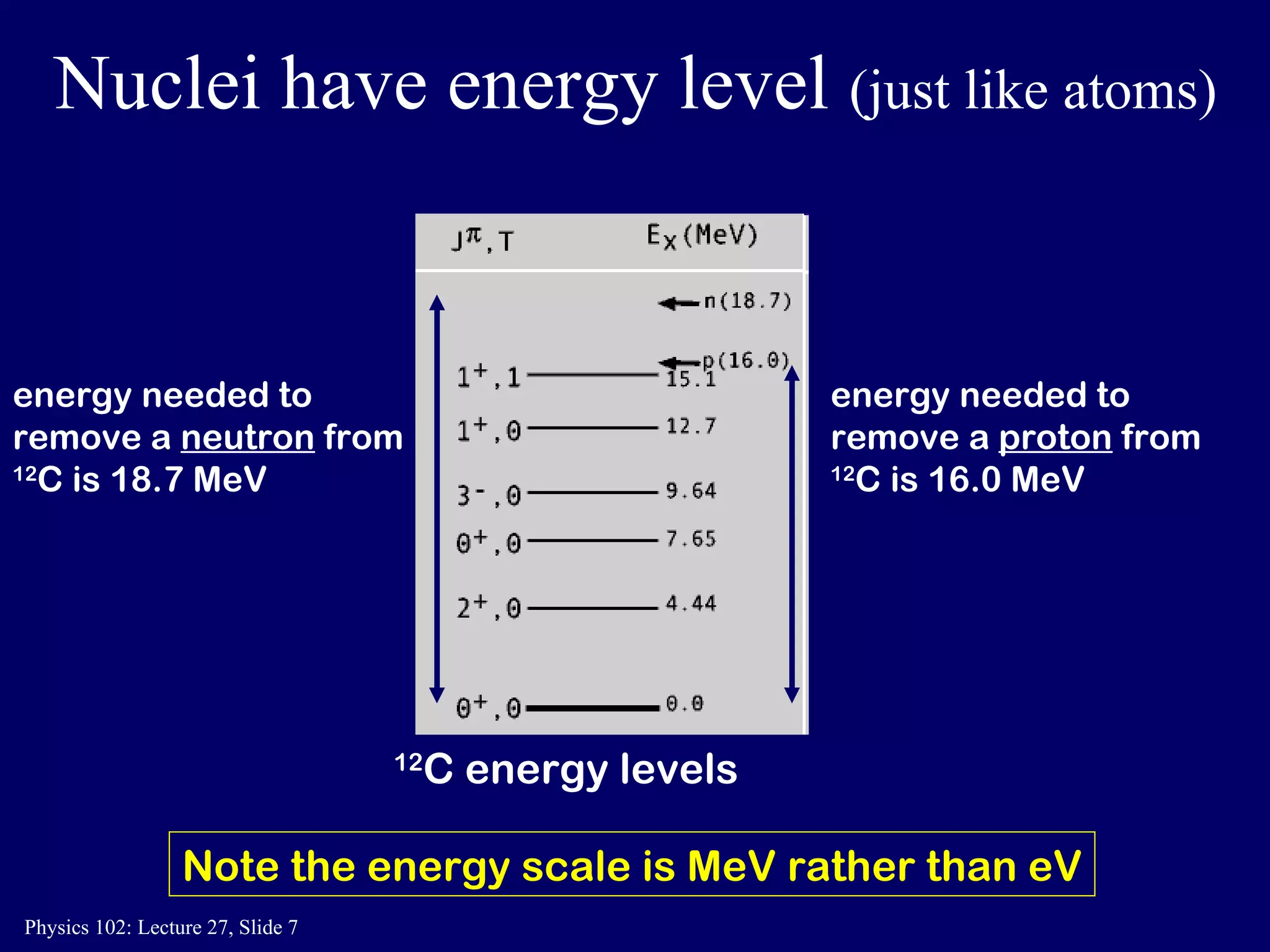
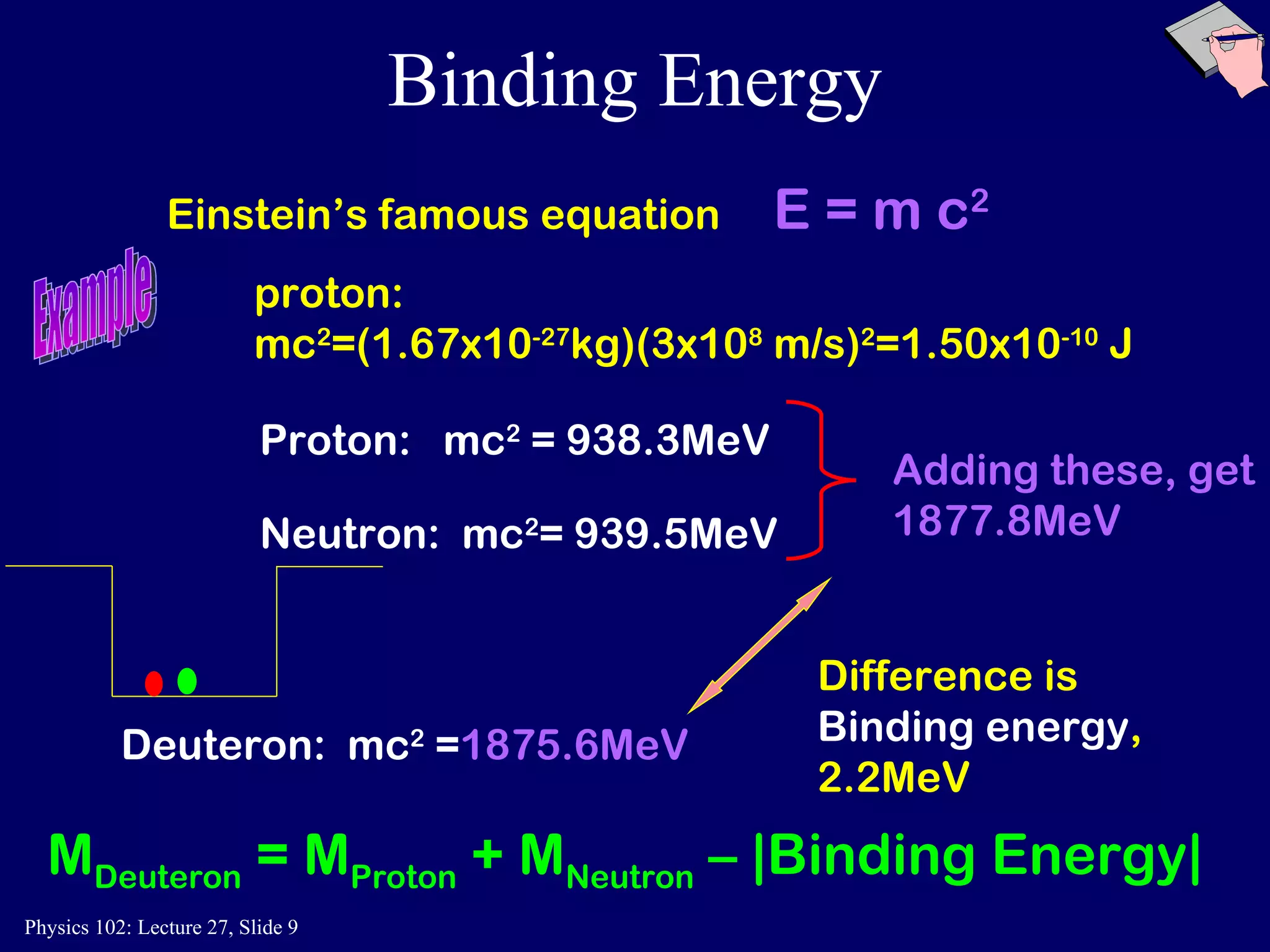
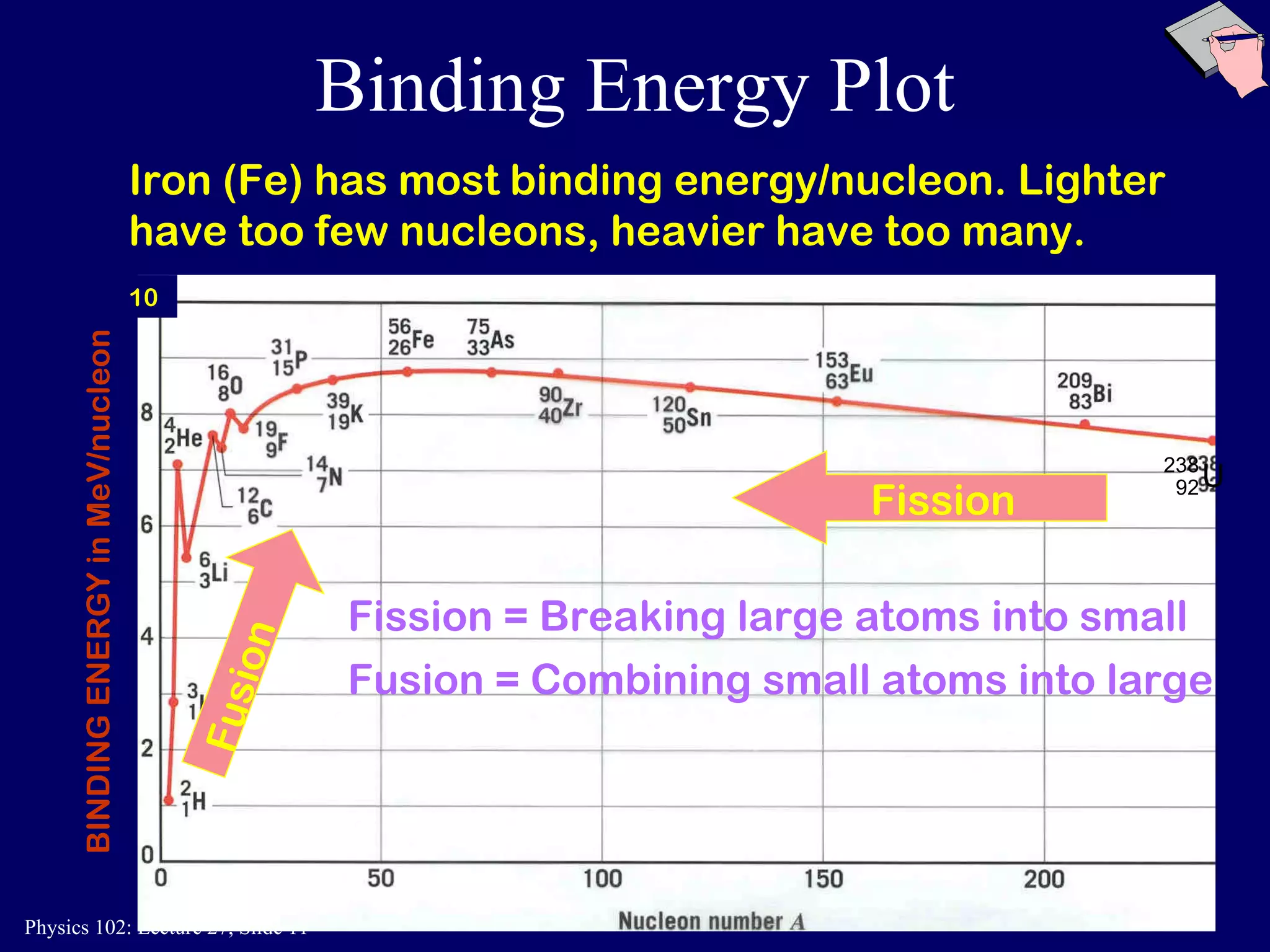
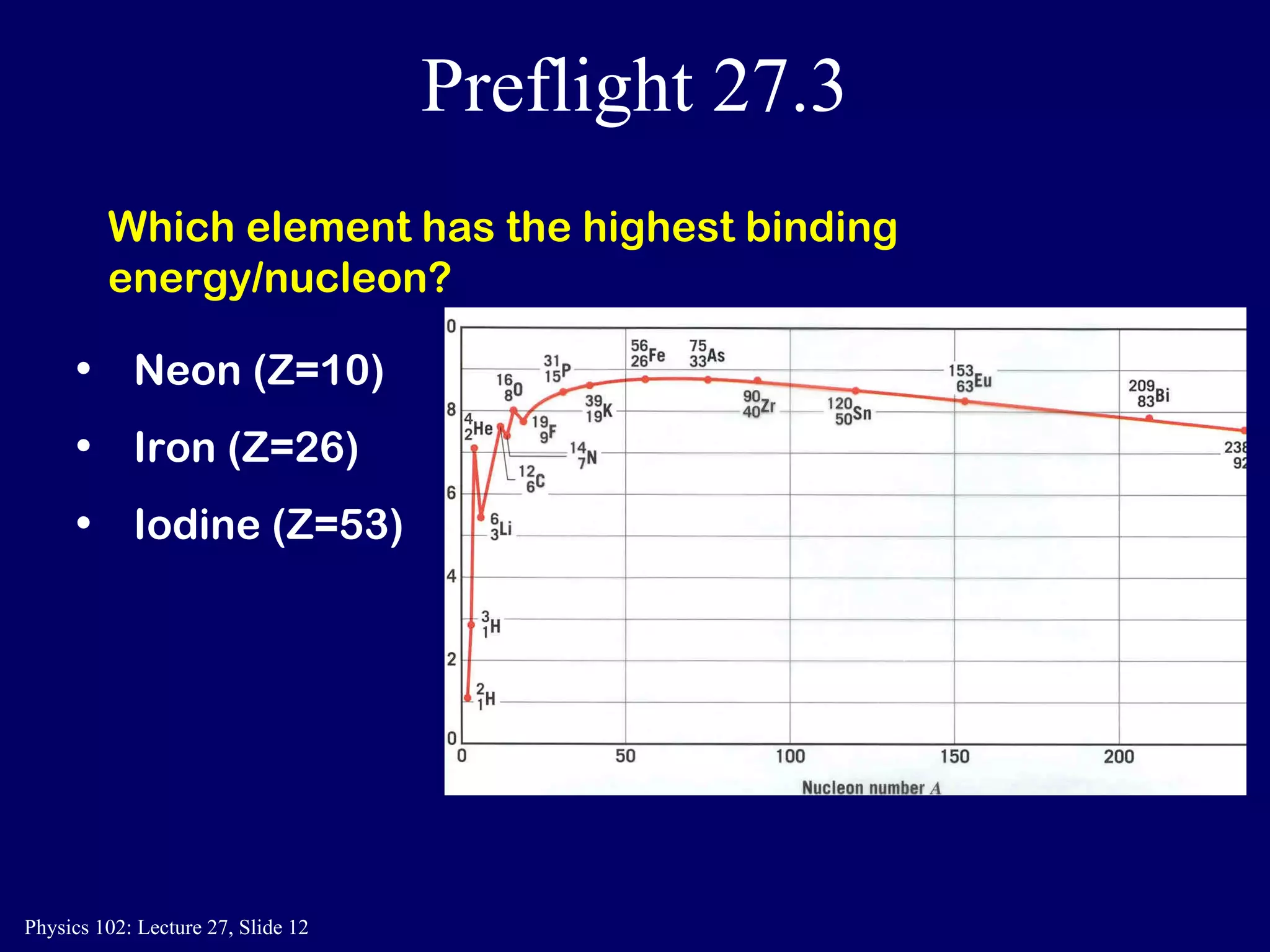
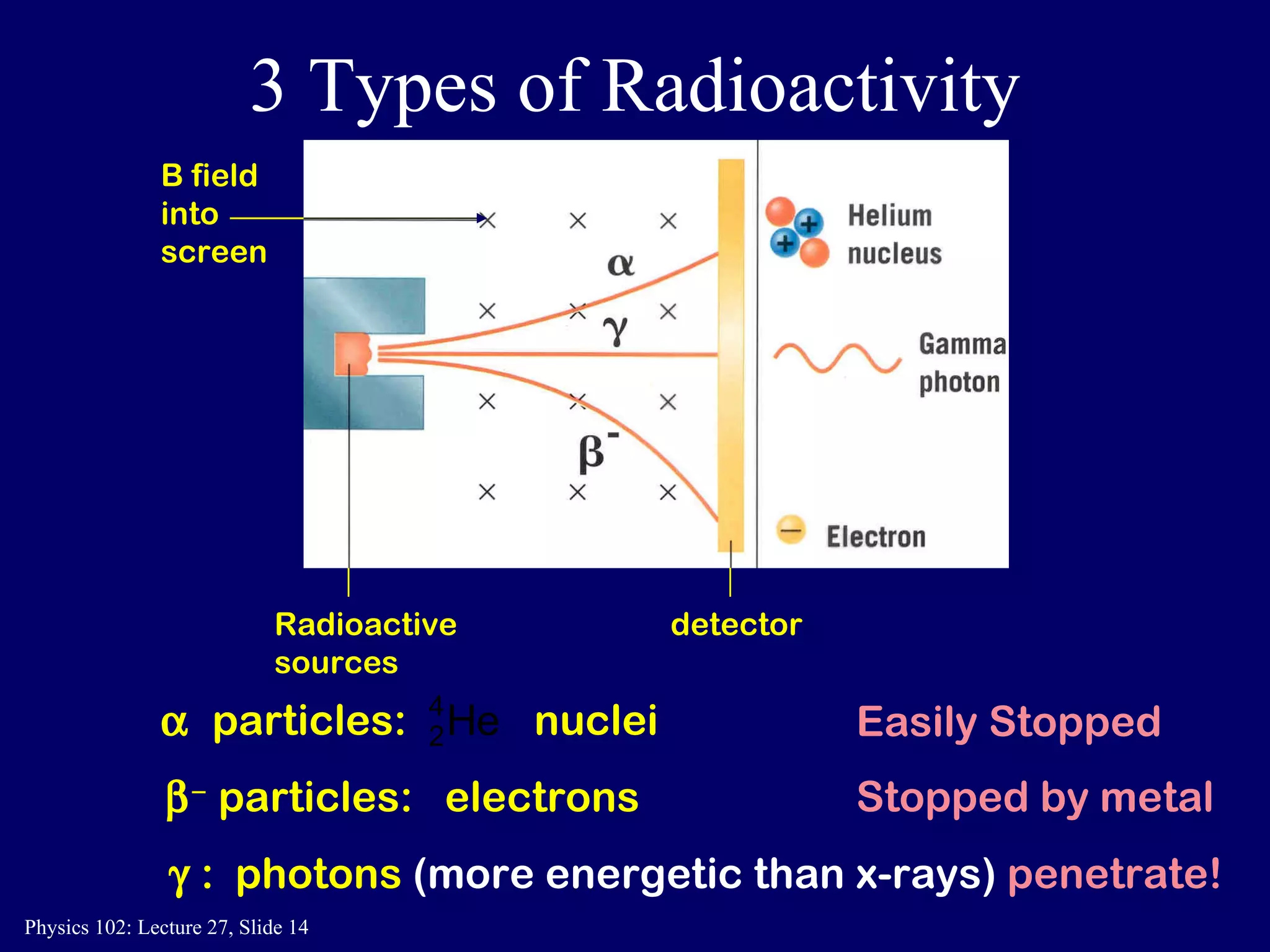
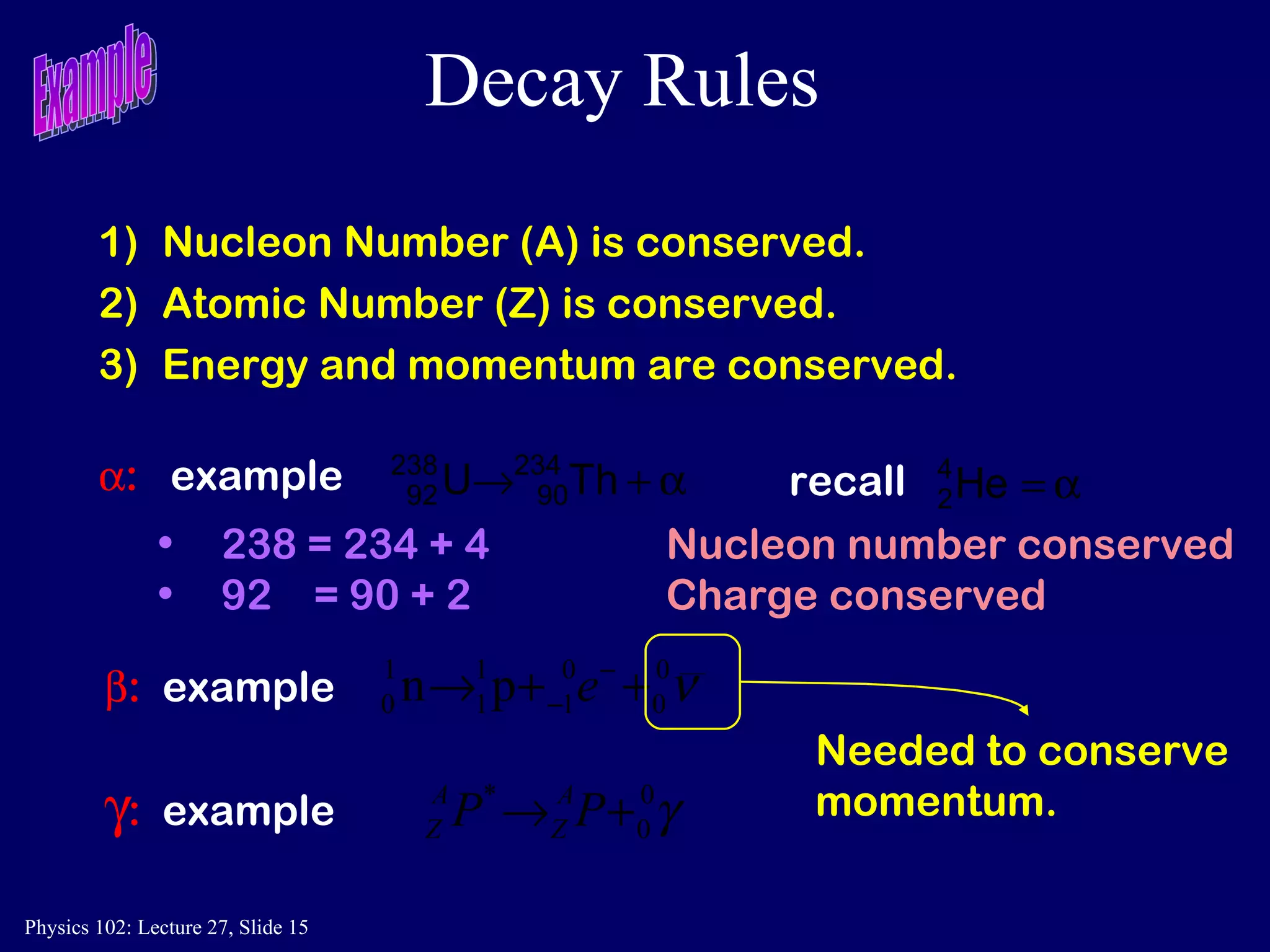
1) Nuclear reactions conserve nucleon number, charge, and energy/momentum. Alpha particles are nuclei, beta particles are electrons, and gamma particles are high-energy photons.
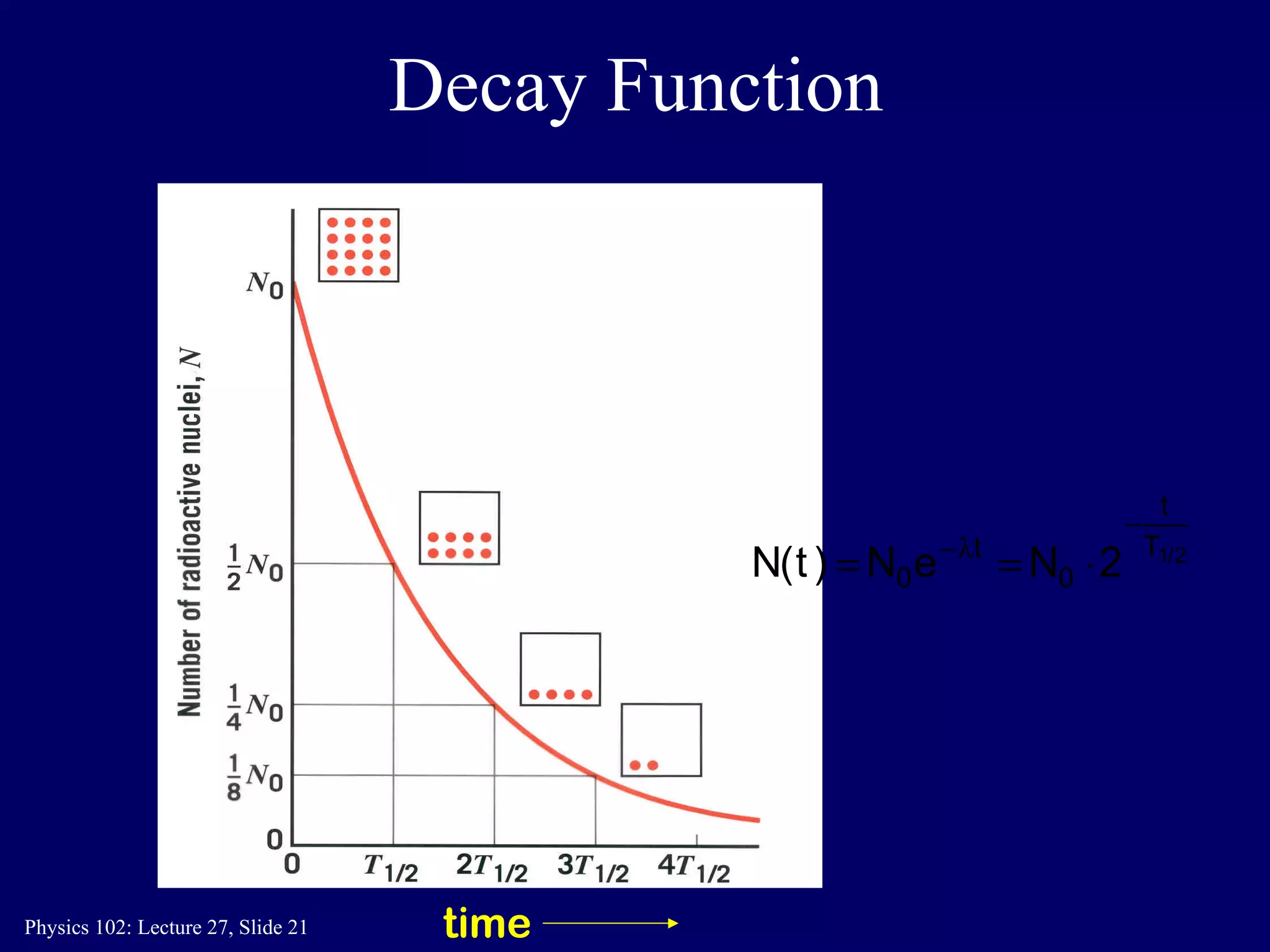
2) Radioactive decay follows first-order kinetics described by half-life, the time for half of the radioactive atoms to decay. Carbon dating determines a sample's age by measuring its remaining radioactive carbon-14.