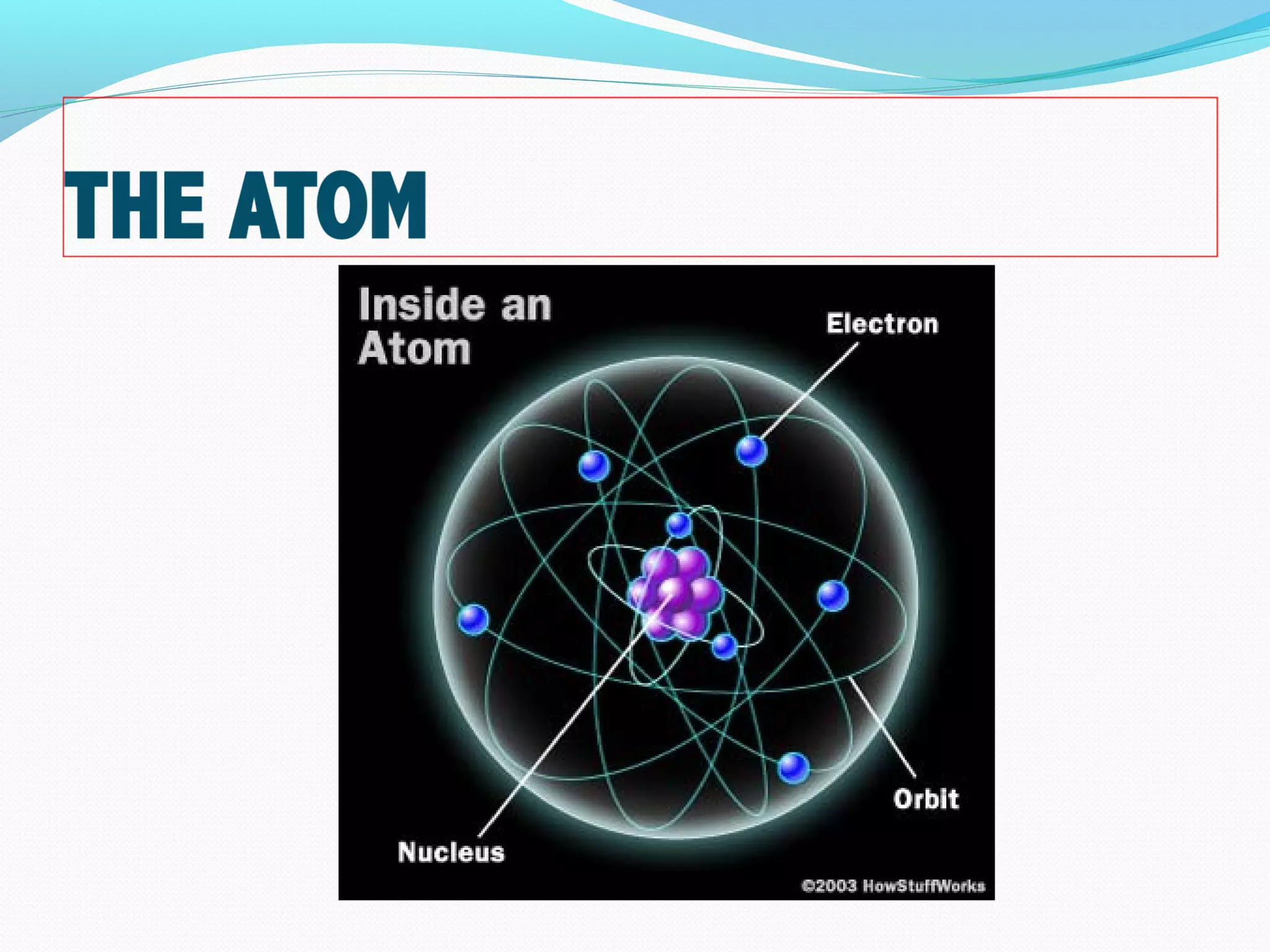
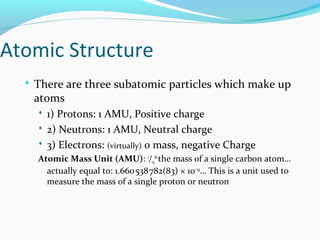
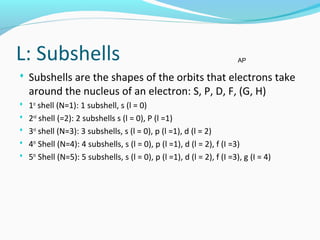
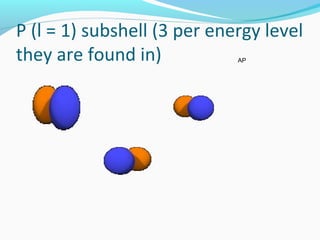
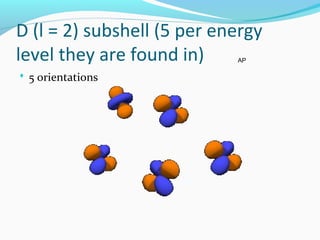
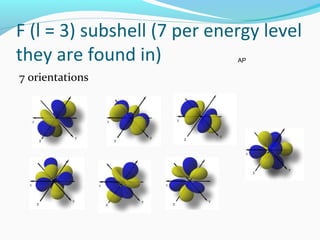
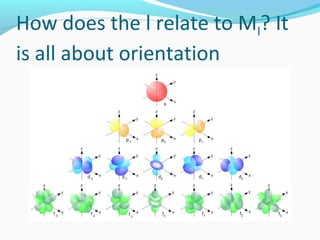
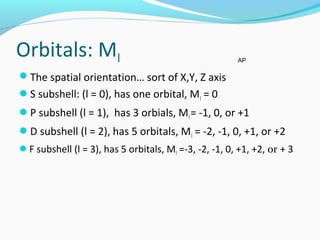
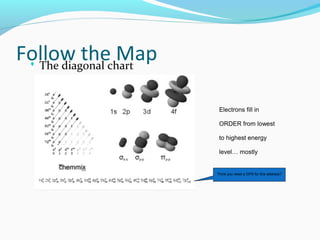
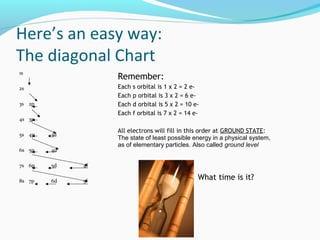
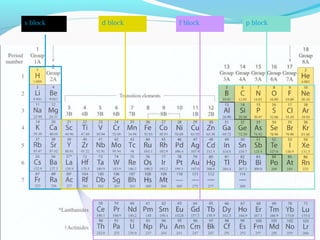
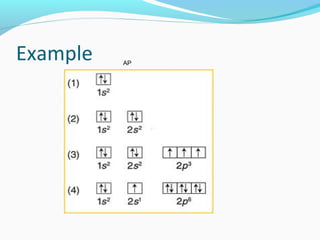
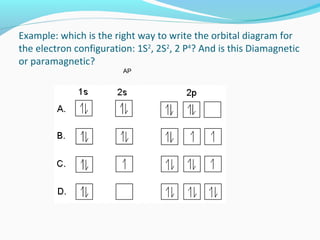
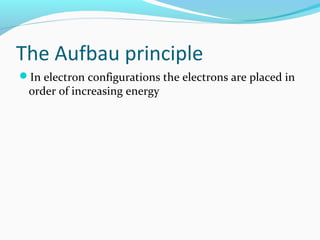
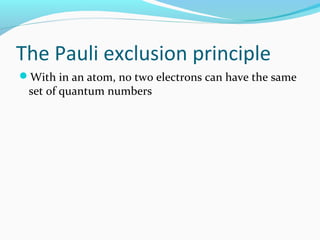
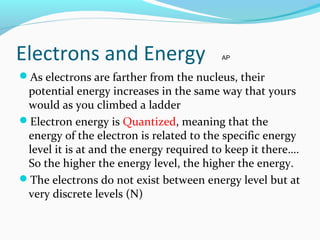
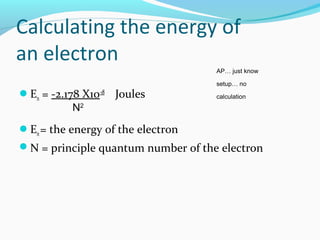
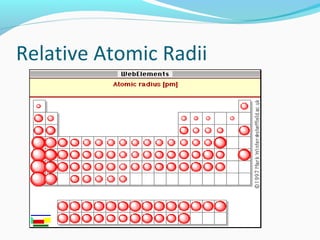
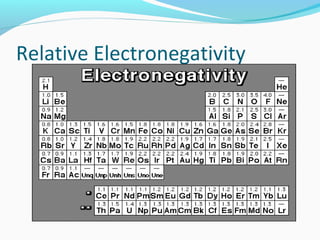
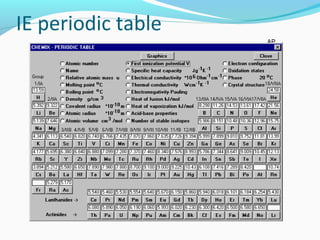
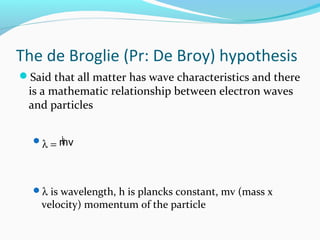
This document discusses atomic structure and properties. It begins by defining the three main subatomic particles - protons, neutrons, and electrons - and their relative masses and charges. It then explains atomic mass units and discusses quantum numbers like principal, azimuthal, magnetic, and spin quantum numbers. The rest of the document covers electron configurations, orbital diagrams, atomic orbitals and subshells, ionization energy, electronegativity, and how these properties relate to an element's position on the periodic table.