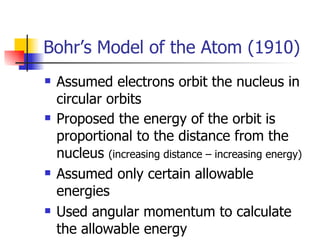
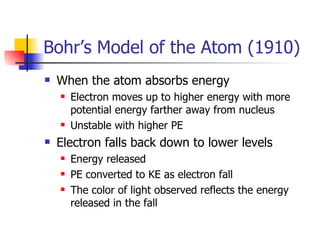
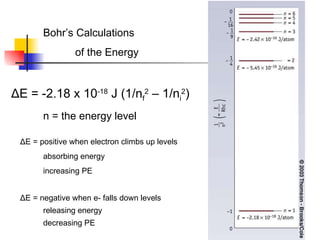
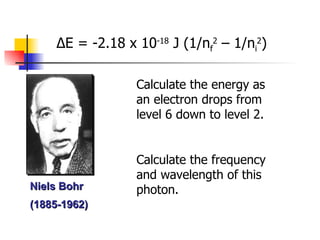
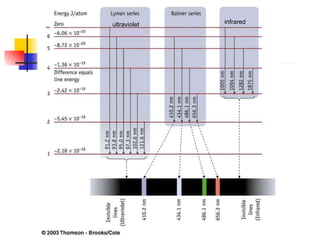
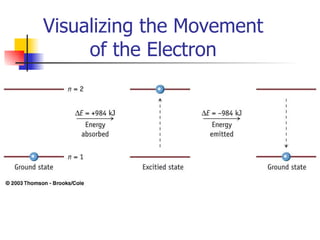
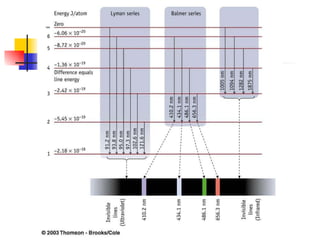
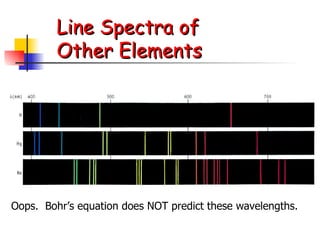
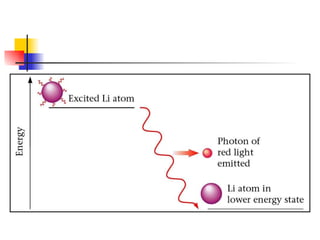
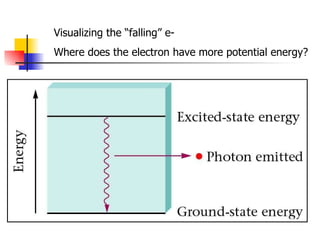
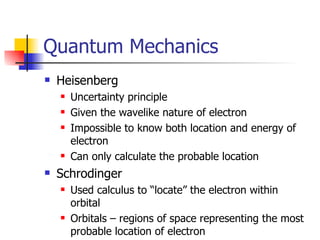
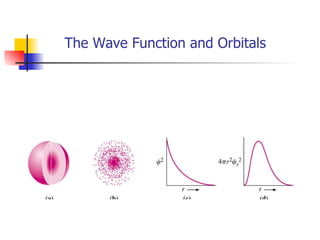
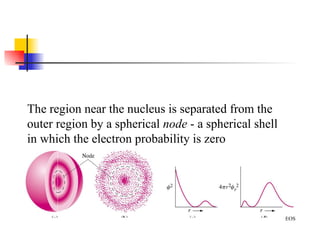
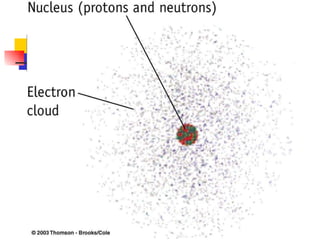
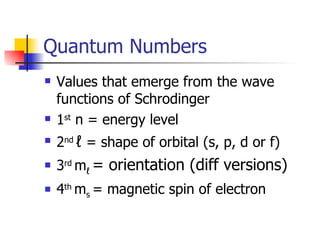
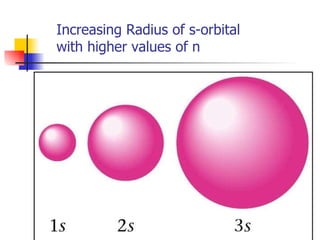
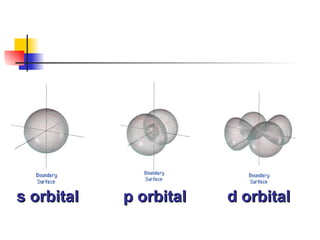
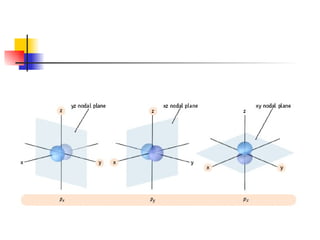
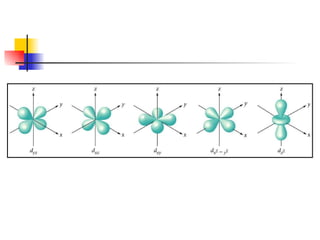
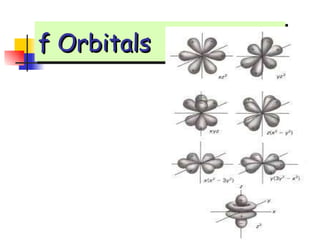
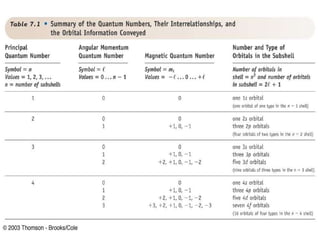
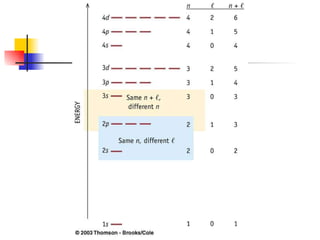
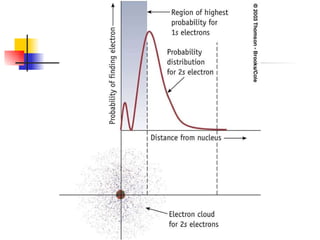
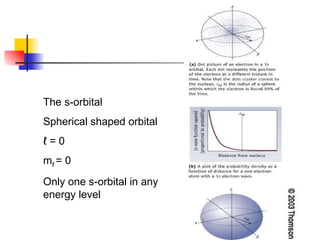
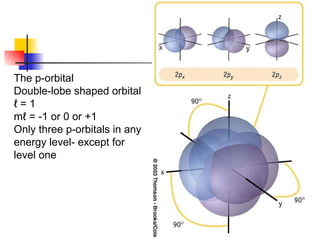
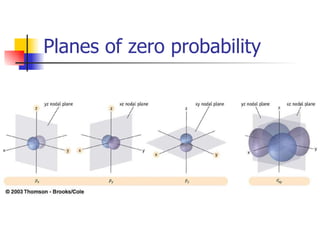
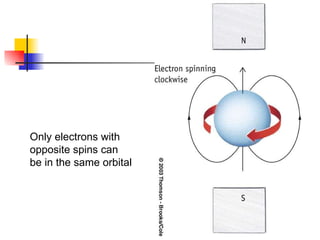
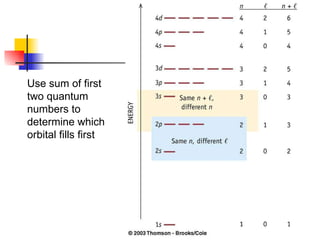
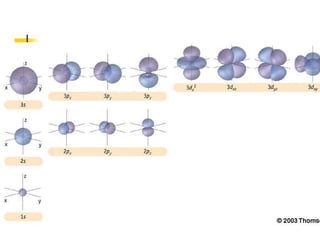
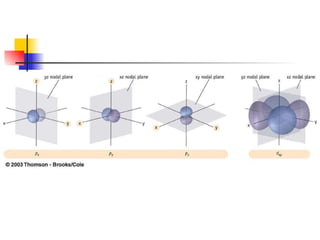
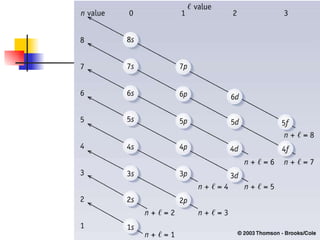
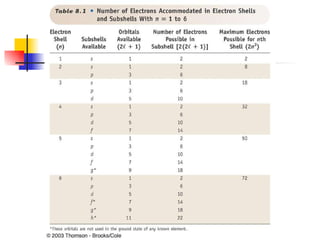
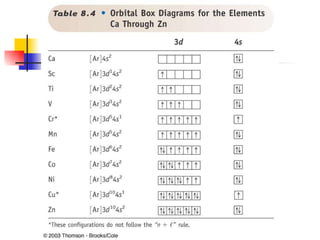
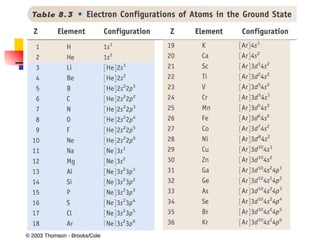
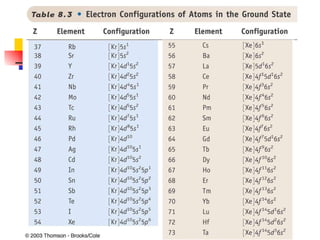
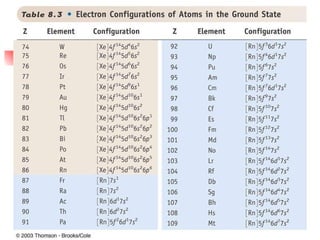
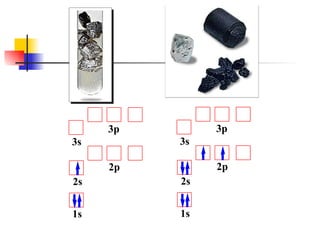
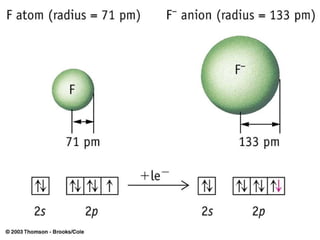
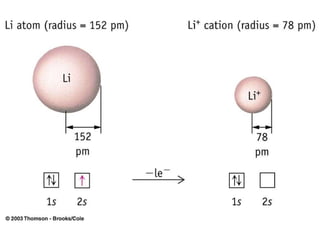
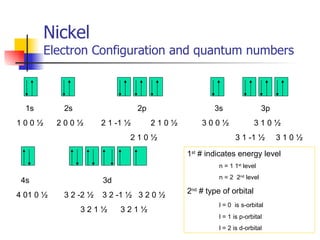
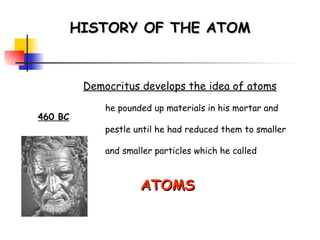
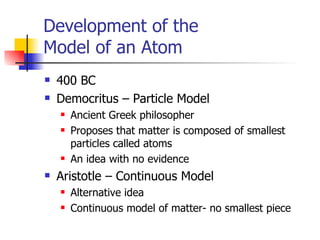
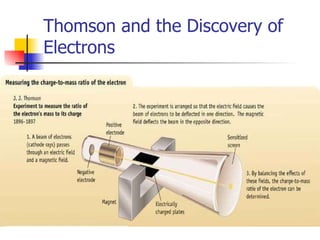
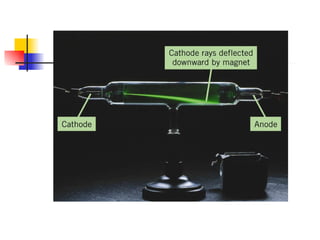
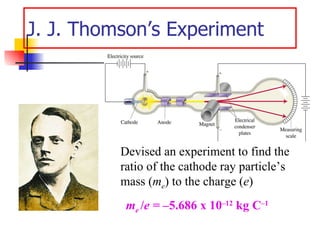
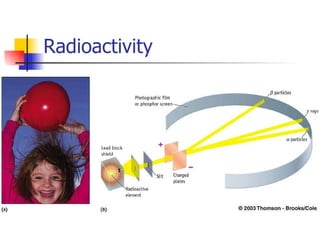
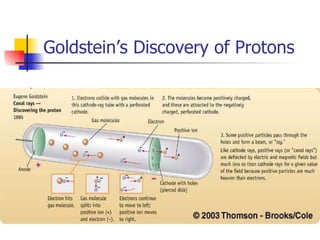
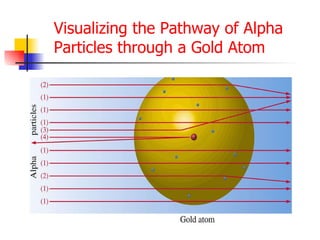
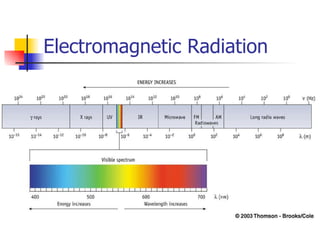
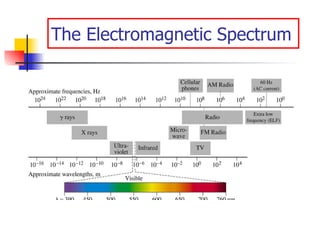
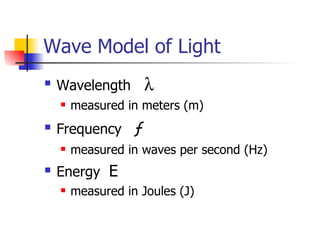
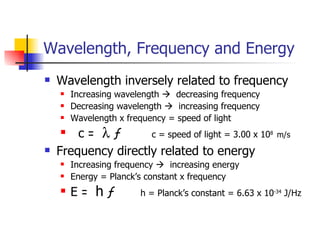
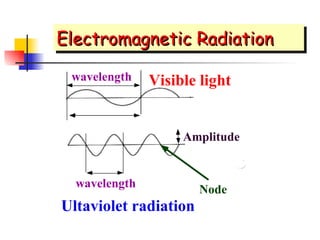
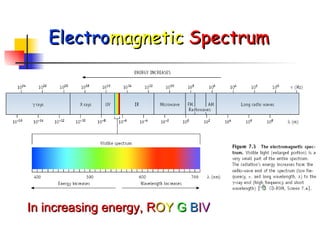
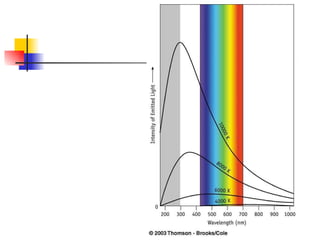
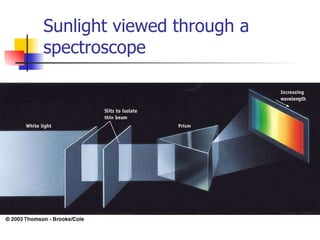
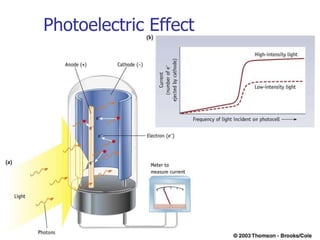
The document provides a history of the development of atomic structure models from ancient Greek philosophers' ideas of indivisible atoms to the modern quantum mechanical model. It describes key experiments and findings such as Thomson's discovery of electrons, Rutherford's gold foil experiment, and Bohr's model of electron orbits that led to modern atomic theory. The emission spectra of elements provided evidence that electrons exist in specific energy levels and orbitals within atoms.




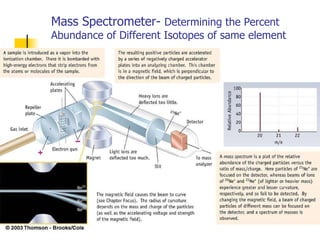
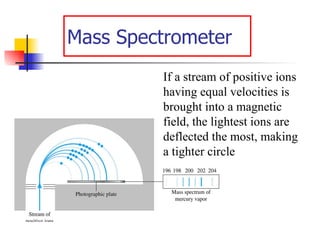
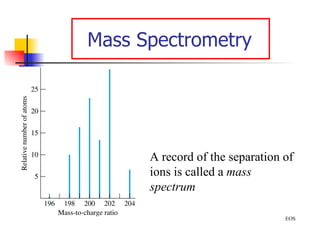

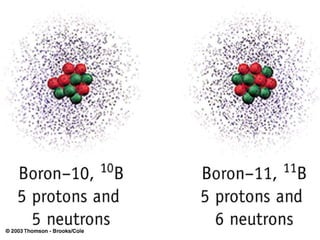
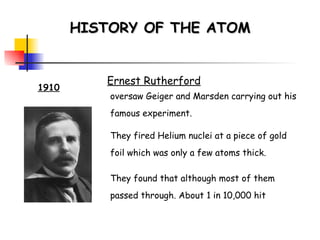
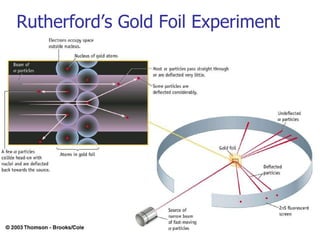
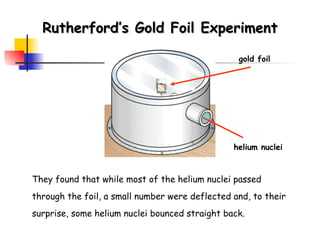









![Rydberg Equation 1/ λ = R H [1/n 1 2 - 1/n 2 2 ] R H = 1.09678 x 10 -2 nm -1 Solve the equation for an electron moving from level 4 to 2.](https://image.slidesharecdn.com/lab9-100222132012-phpapp02/85/Lab-8-atomic-structure-46-320.jpg)