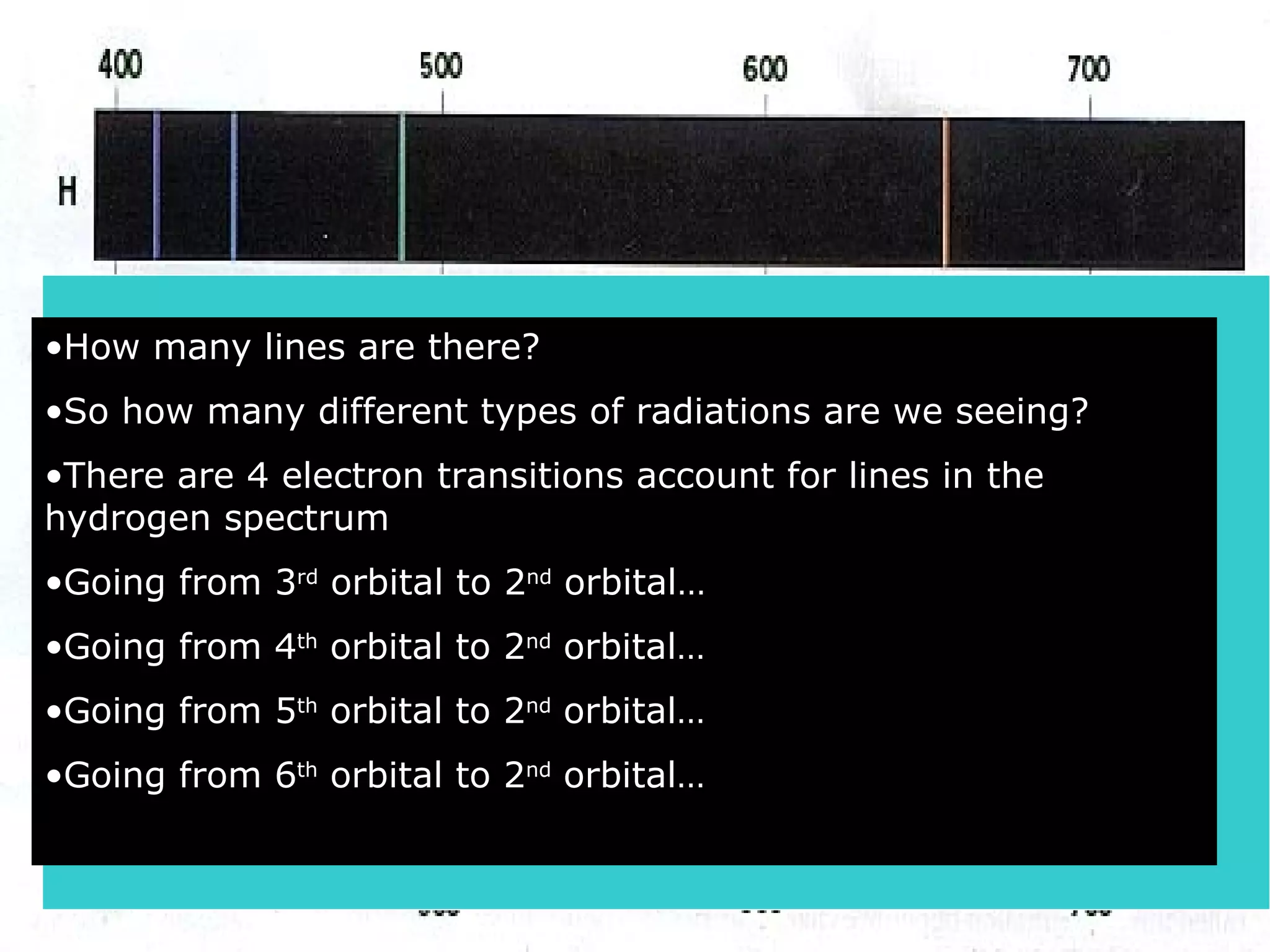
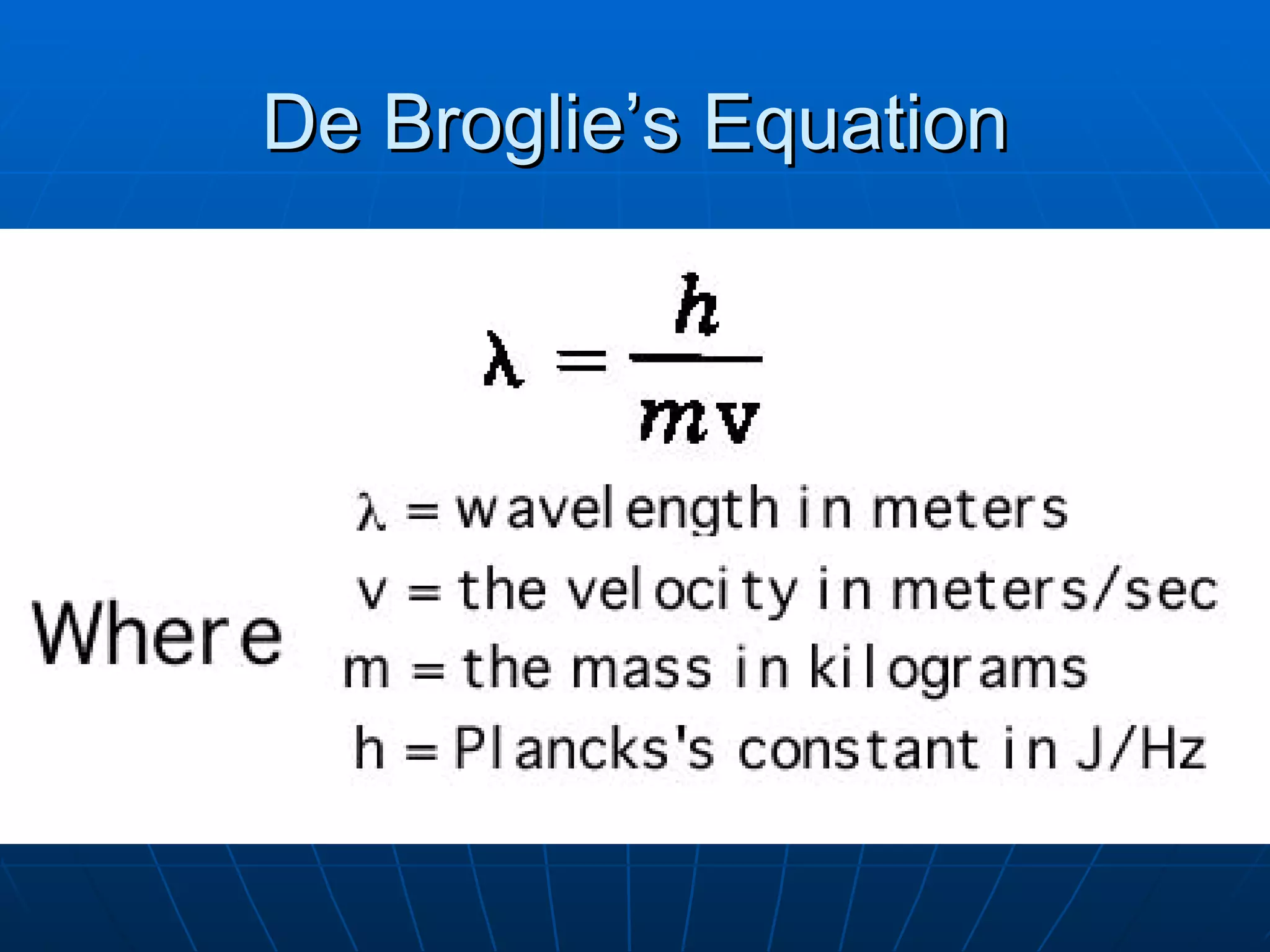
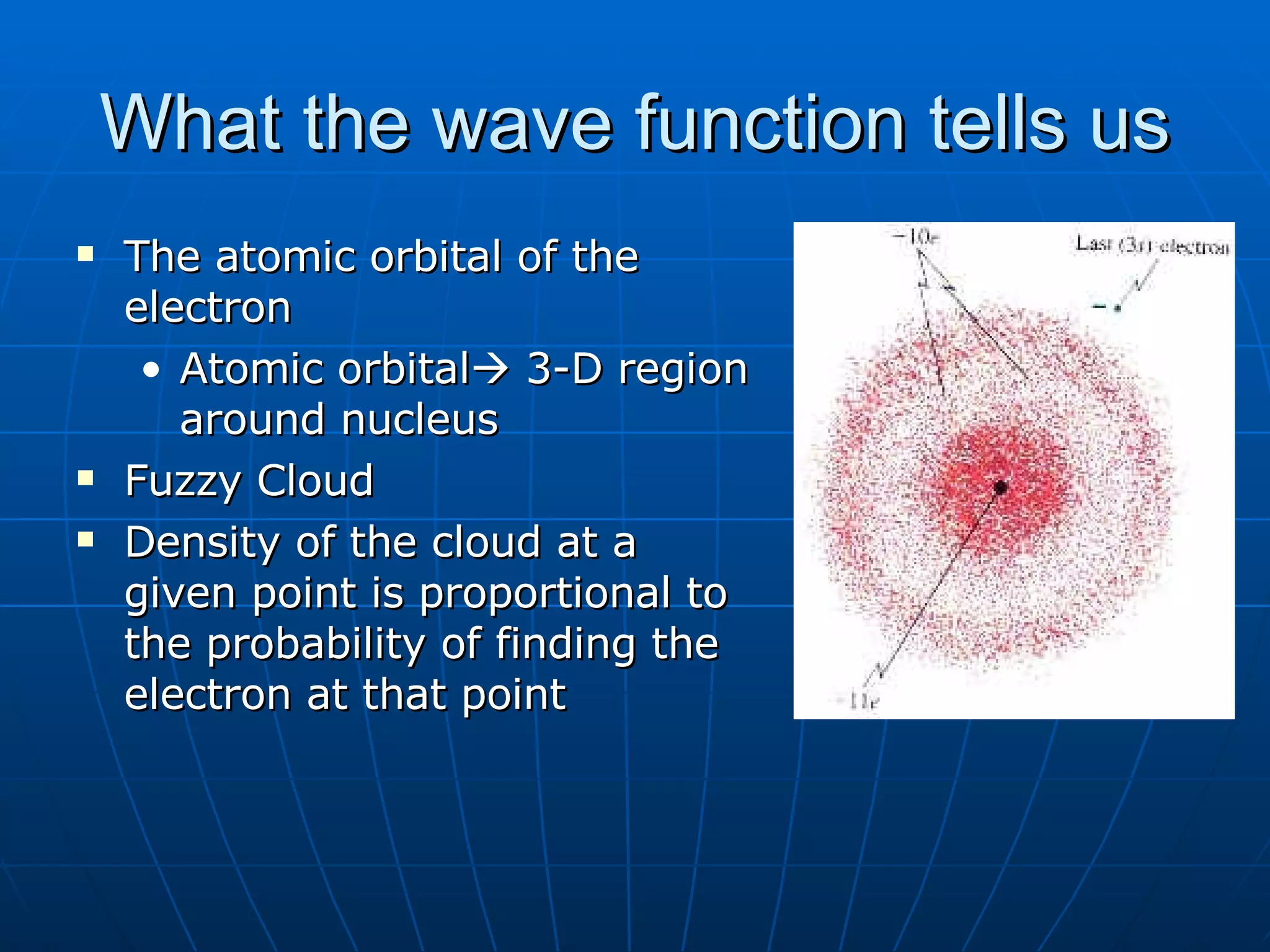
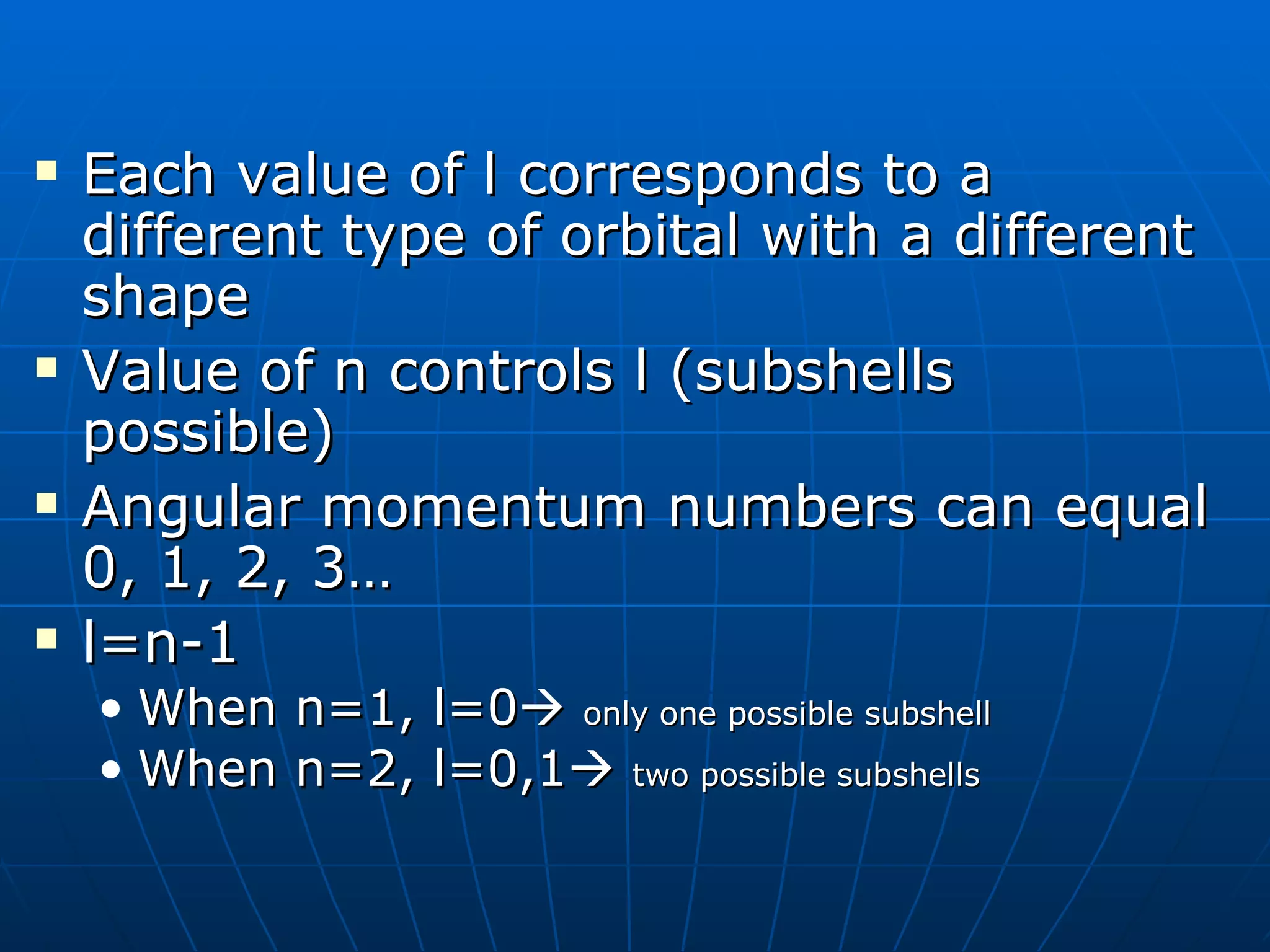
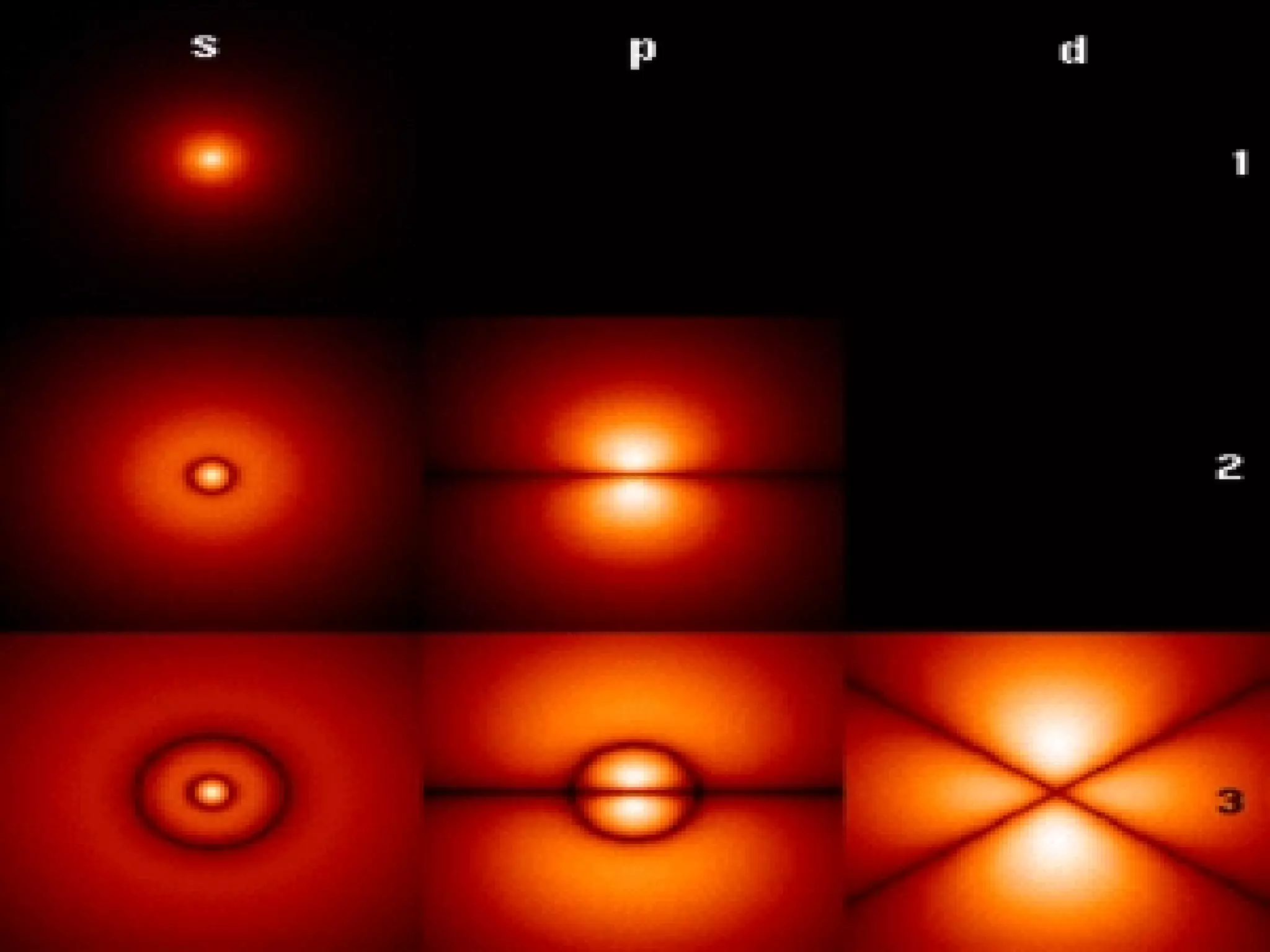
1. The quantum mechanical model of the atom describes electrons as existing in orbitals defined by four quantum numbers rather than following circular orbits.
2. The quantum numbers specify the principal energy level, subshell type, orientation in space, and spin of each electron.
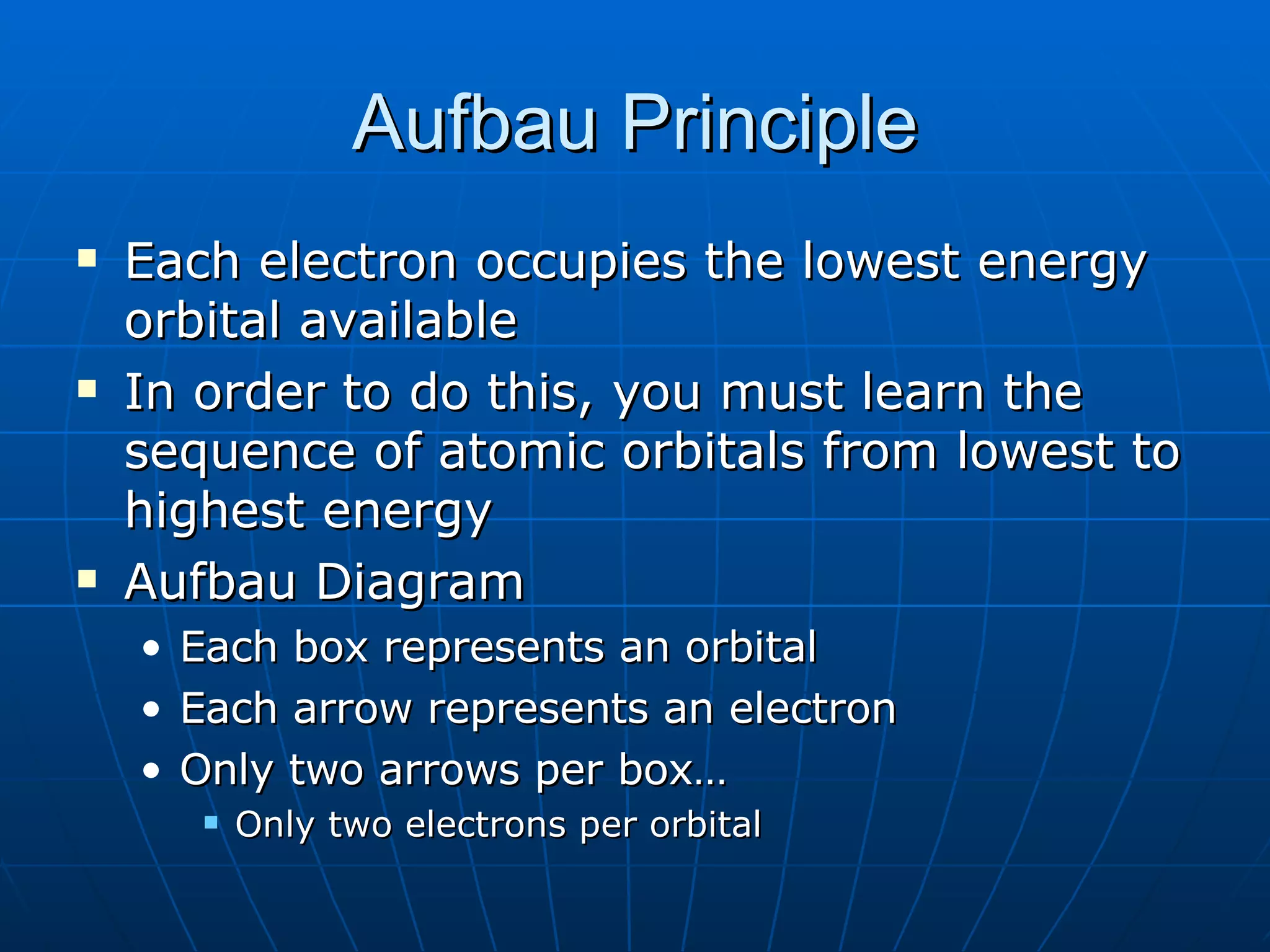
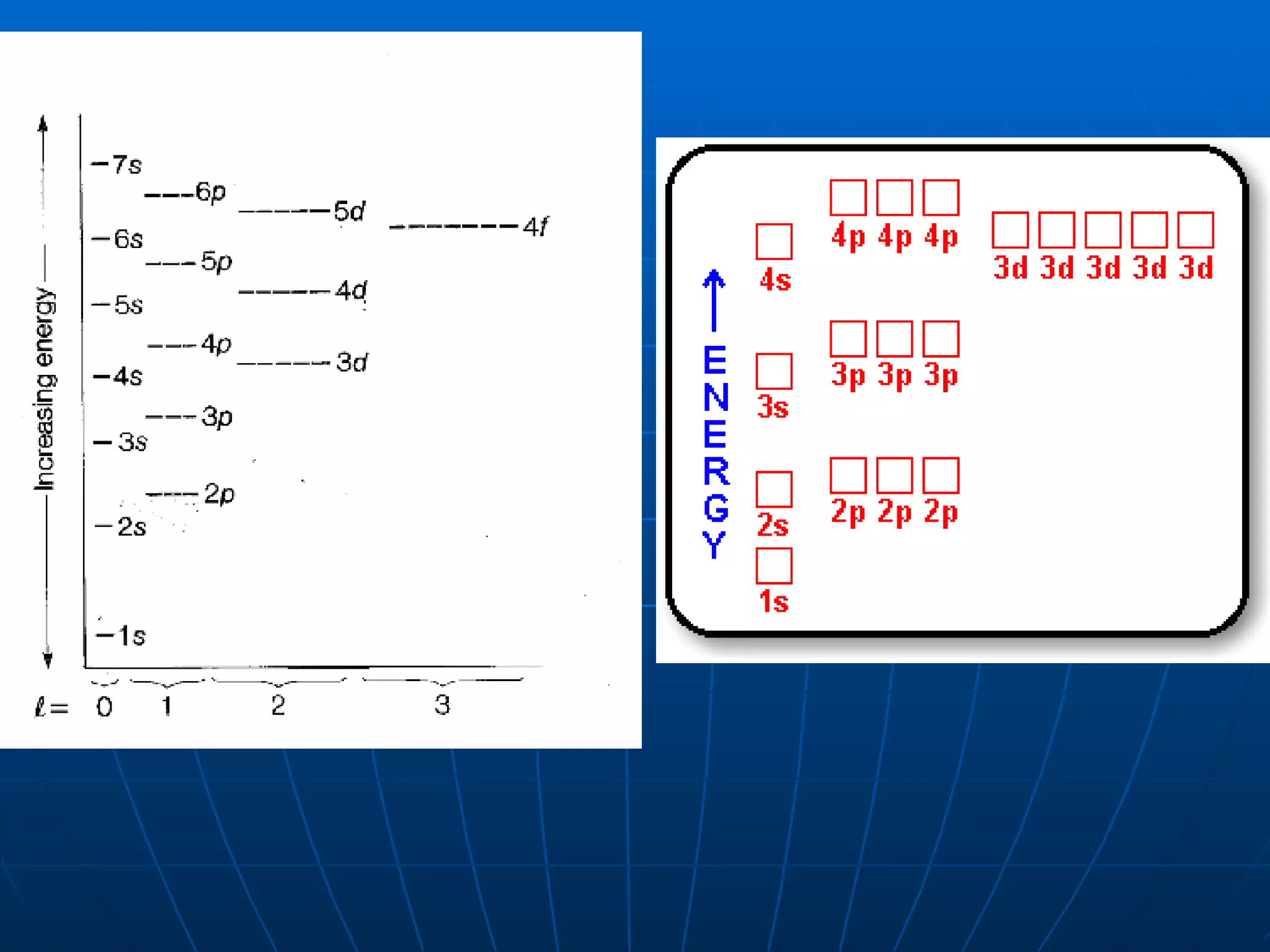
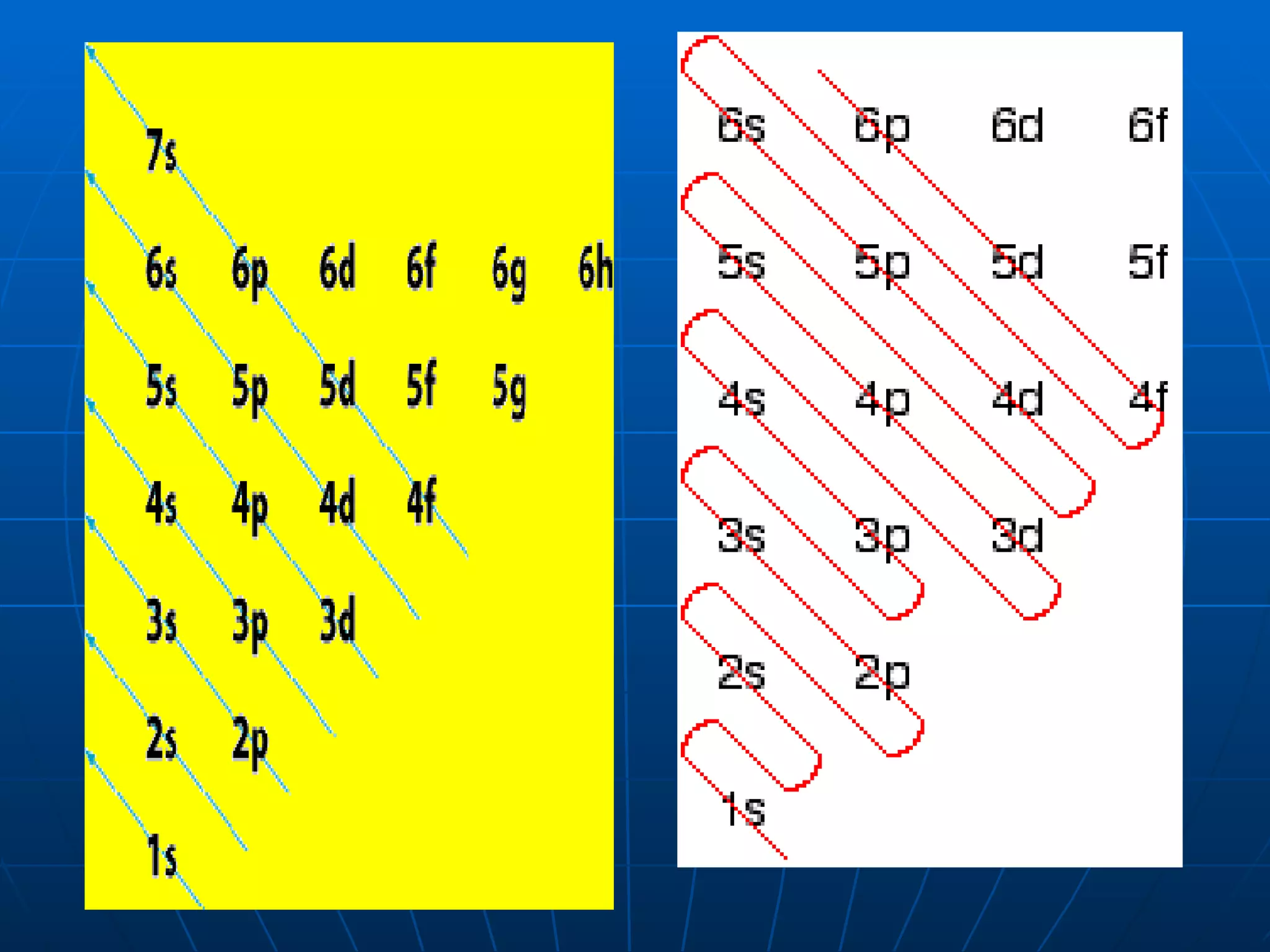
3. Electrons fill atomic orbitals according to increasing energy levels, with each level and sublevel having a fixed capacity for electrons.