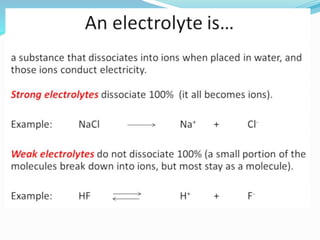
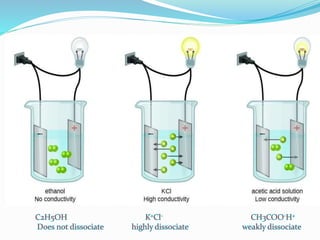
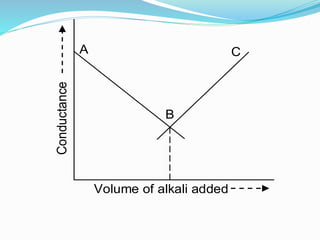
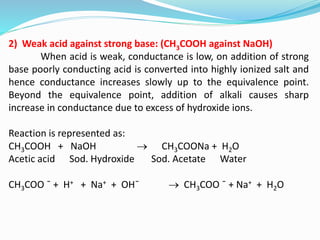
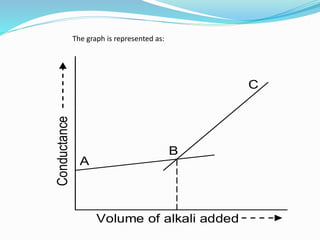
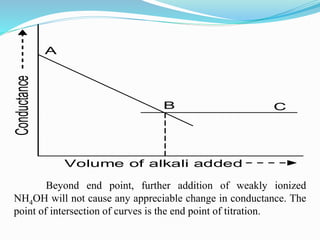
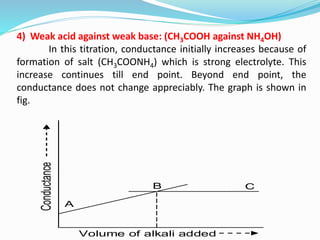
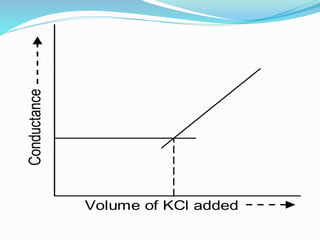
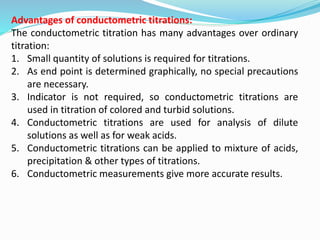
The document discusses conductometric titrations, explaining how the endpoint is determined by measuring the change in conductance of a solution as reagents are added. It includes examples of different types of titrations, such as strong acid against strong base, weak acid against strong base, and precipitation titrations, while highlighting the advantages of conductometric methods over traditional titrations. Advantages include requiring smaller solution quantities, eliminating the need for indicators, and improving accuracy for various titration types.