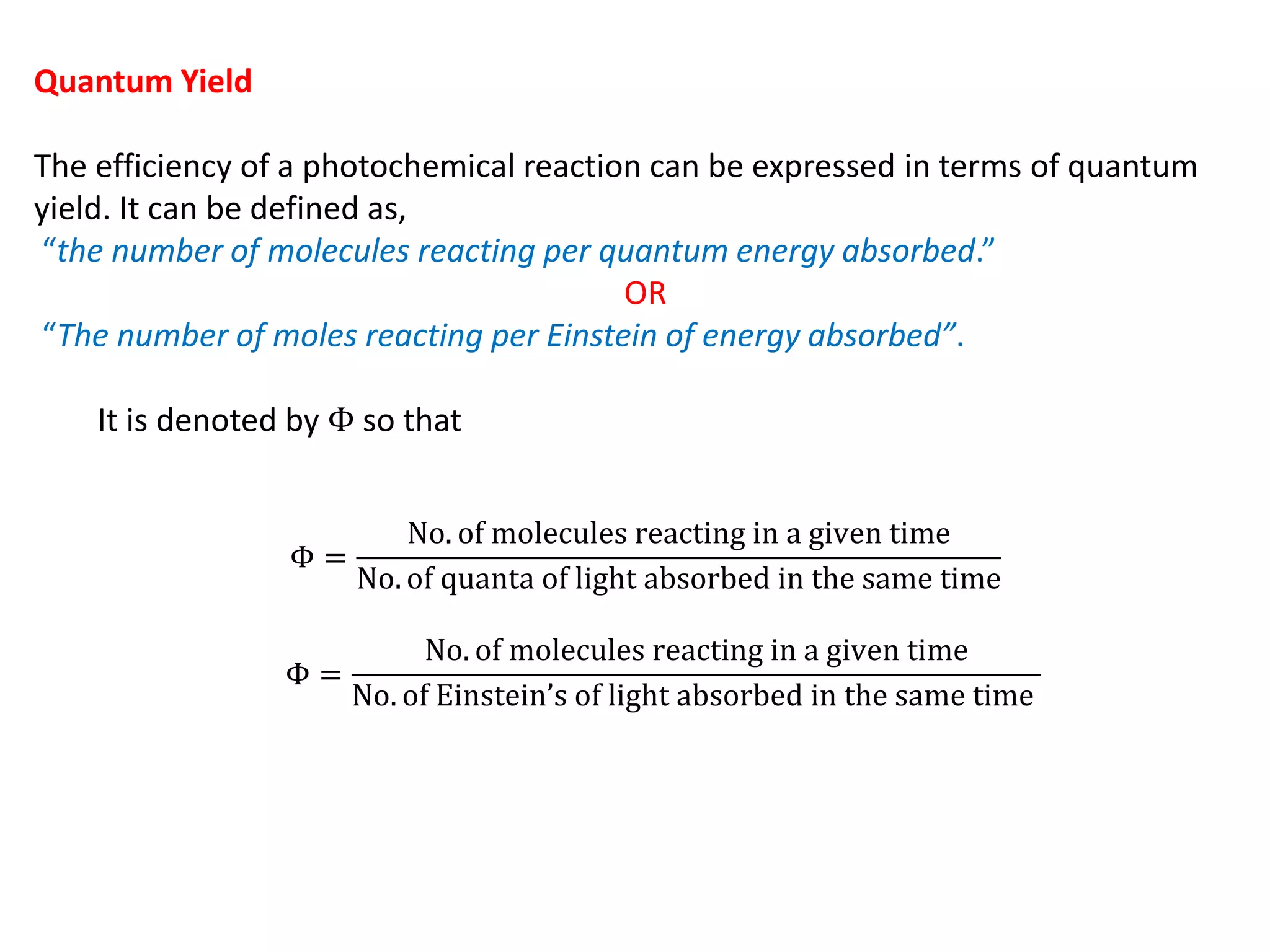
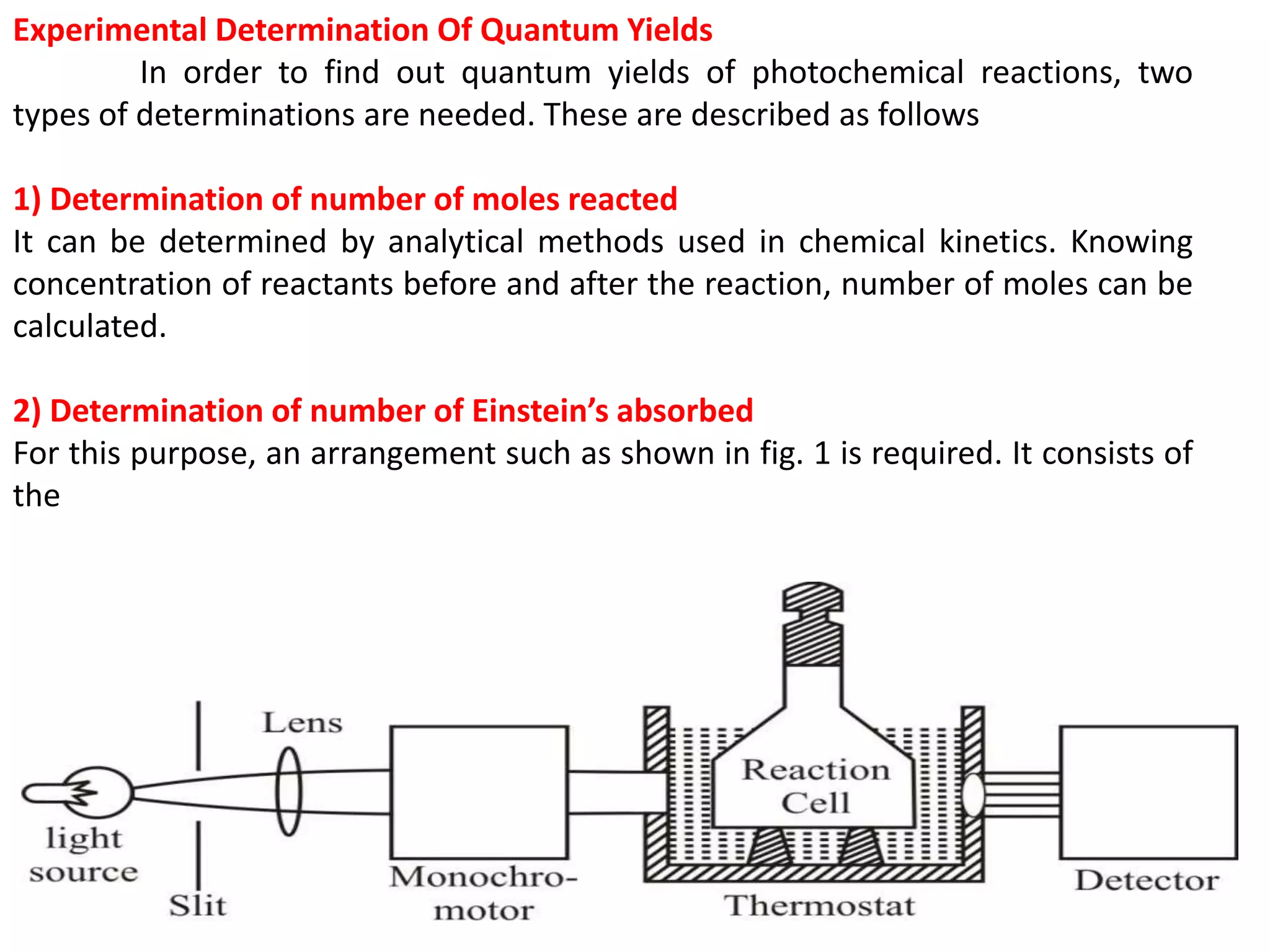
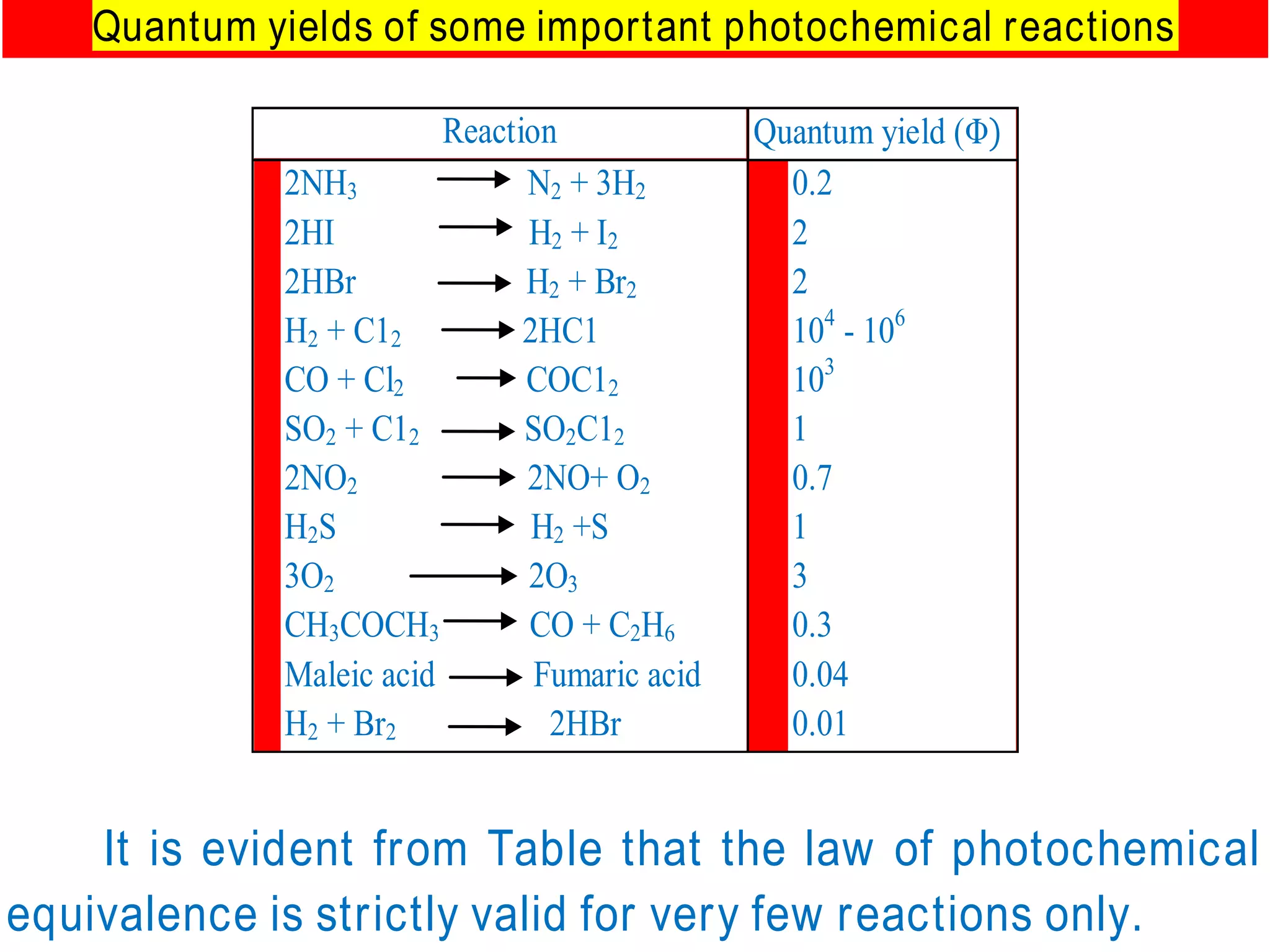
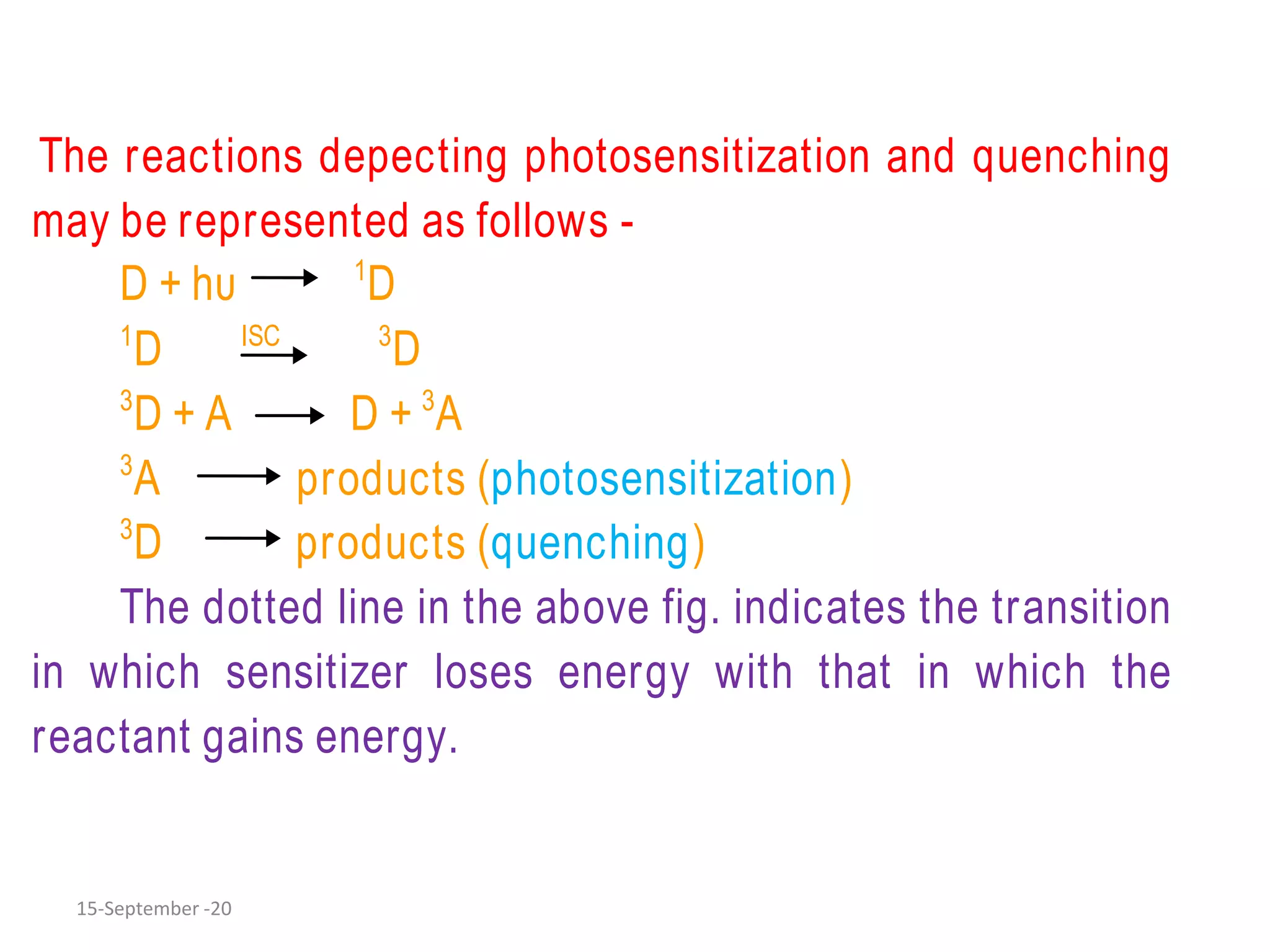
The document discusses quantum yield in photochemical reactions, defining it as the number of molecules reacting per quantum energy absorbed. It outlines methods for determining quantum yield, the types of photochemical reactions, and reasons for variations in quantum yield. Additionally, it explains photosensitized reactions, where a third substance aids in energy transfer to activate reactants not absorbing required radiation.