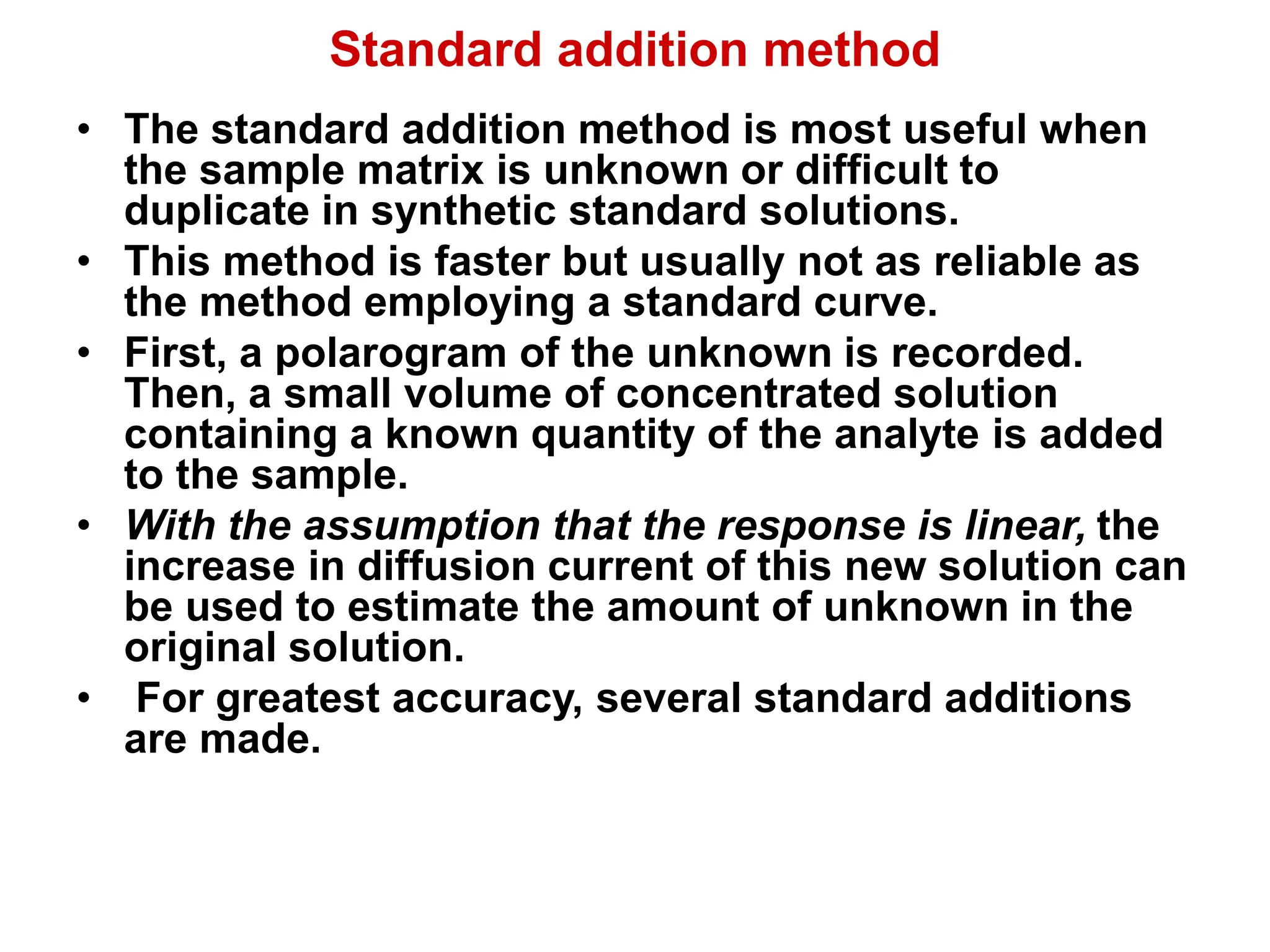
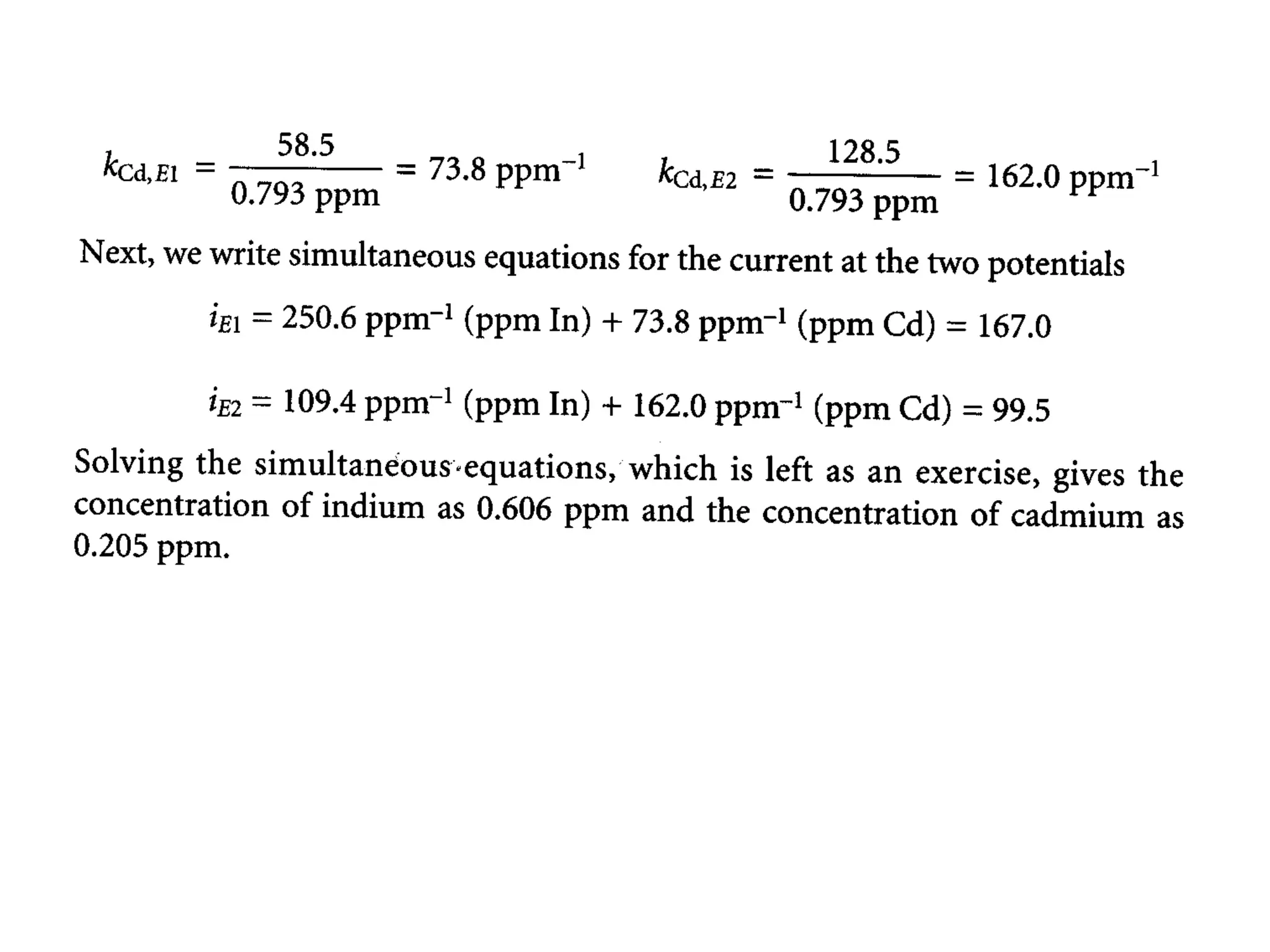
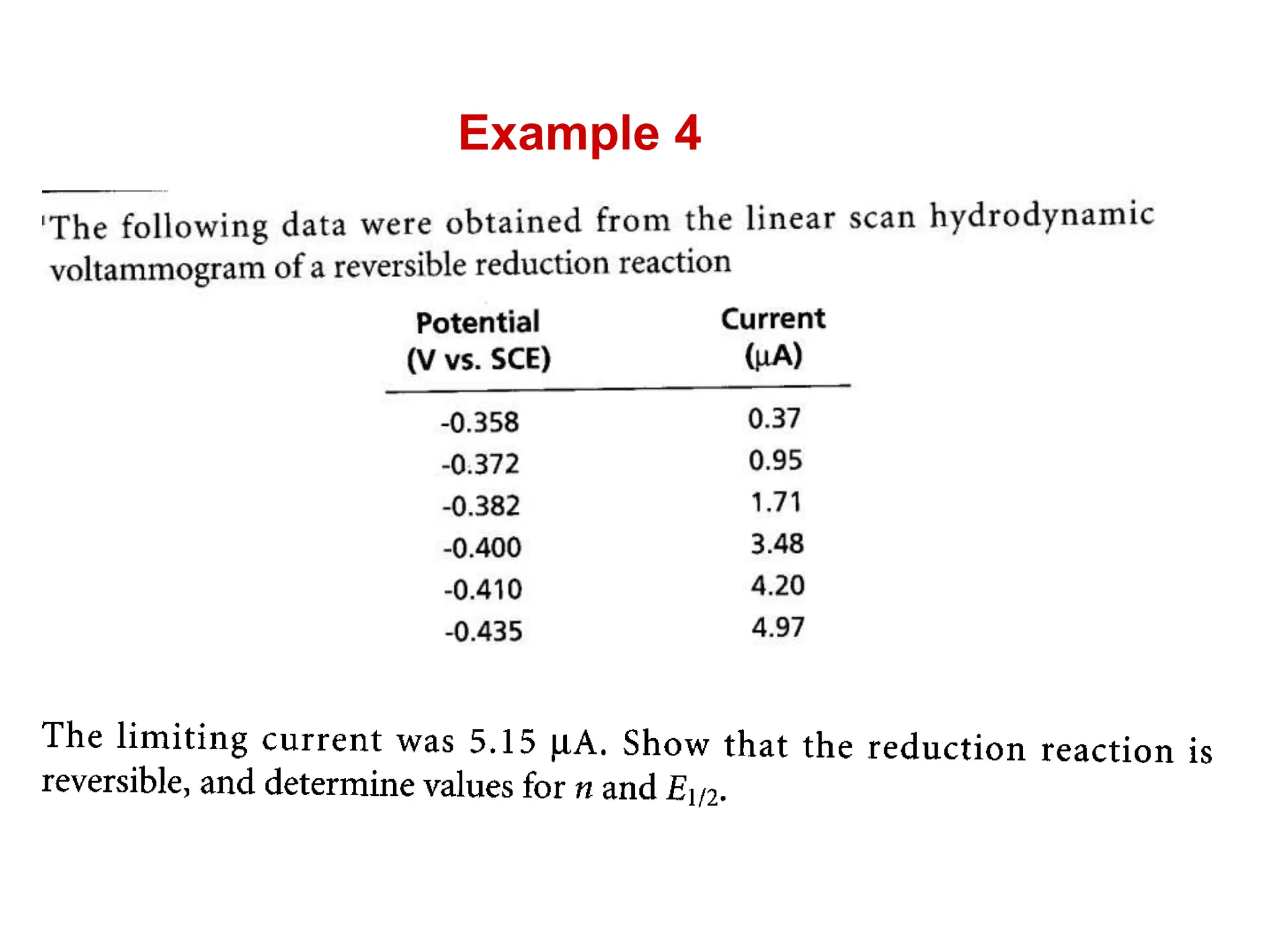
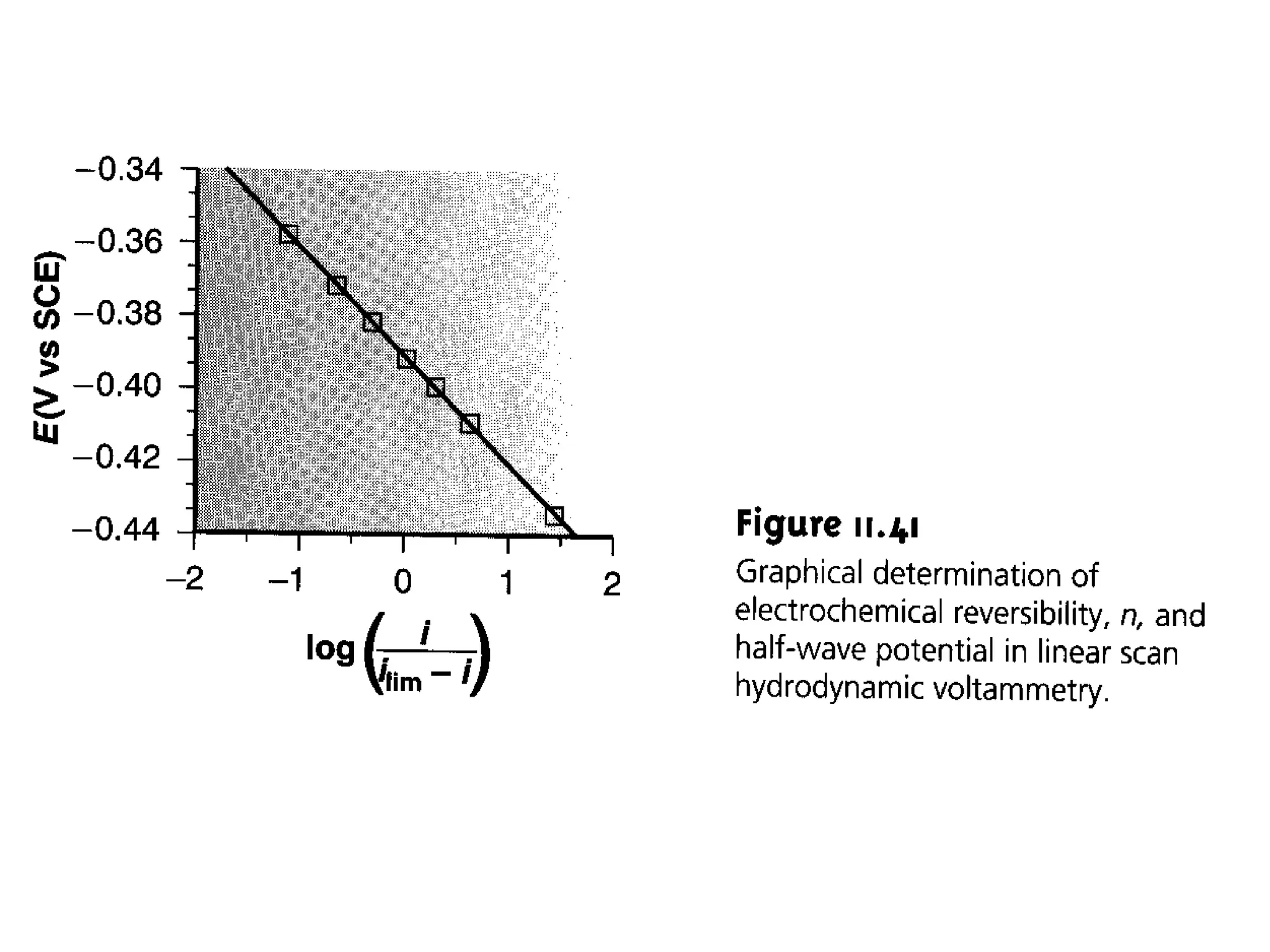
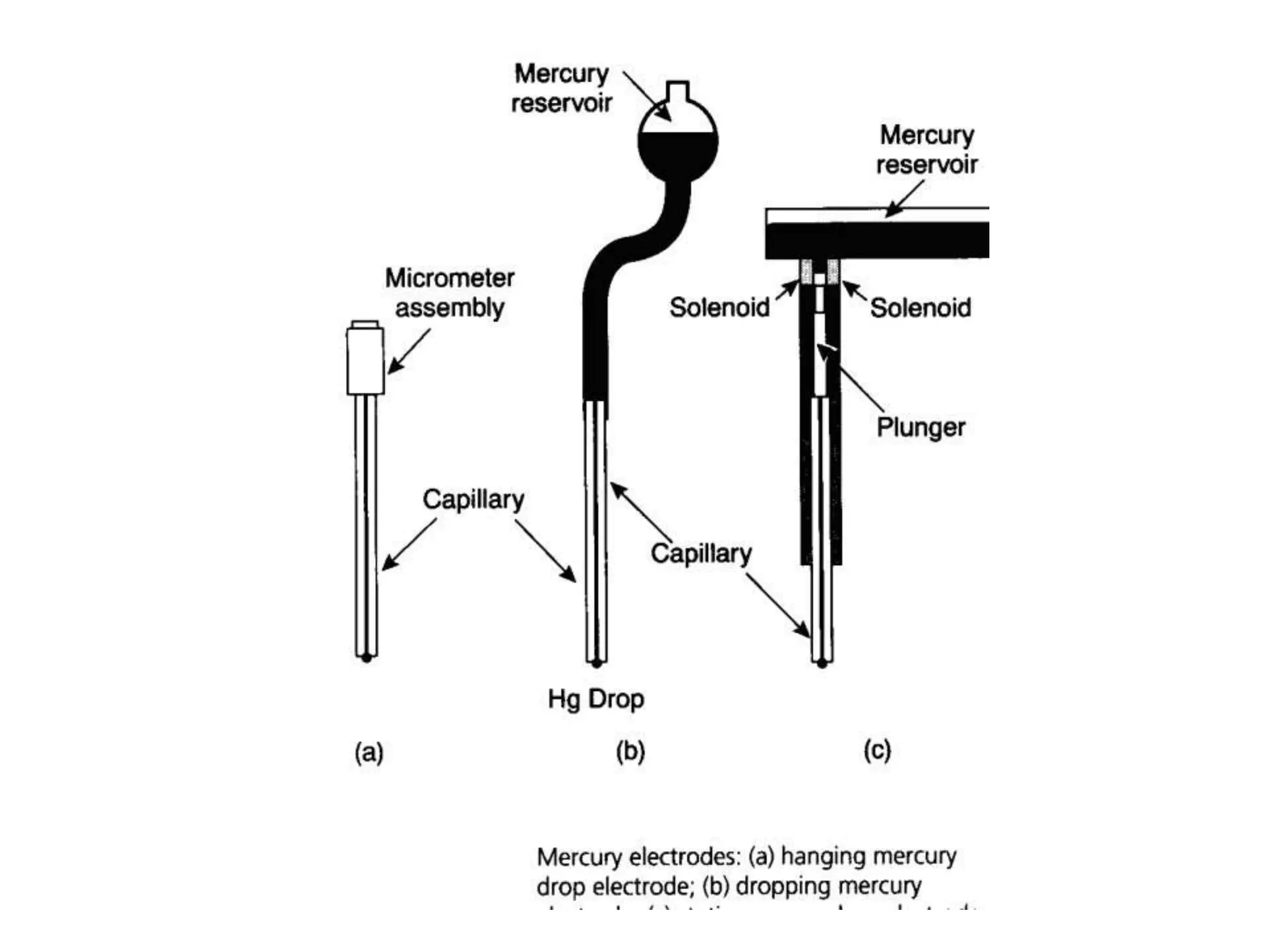
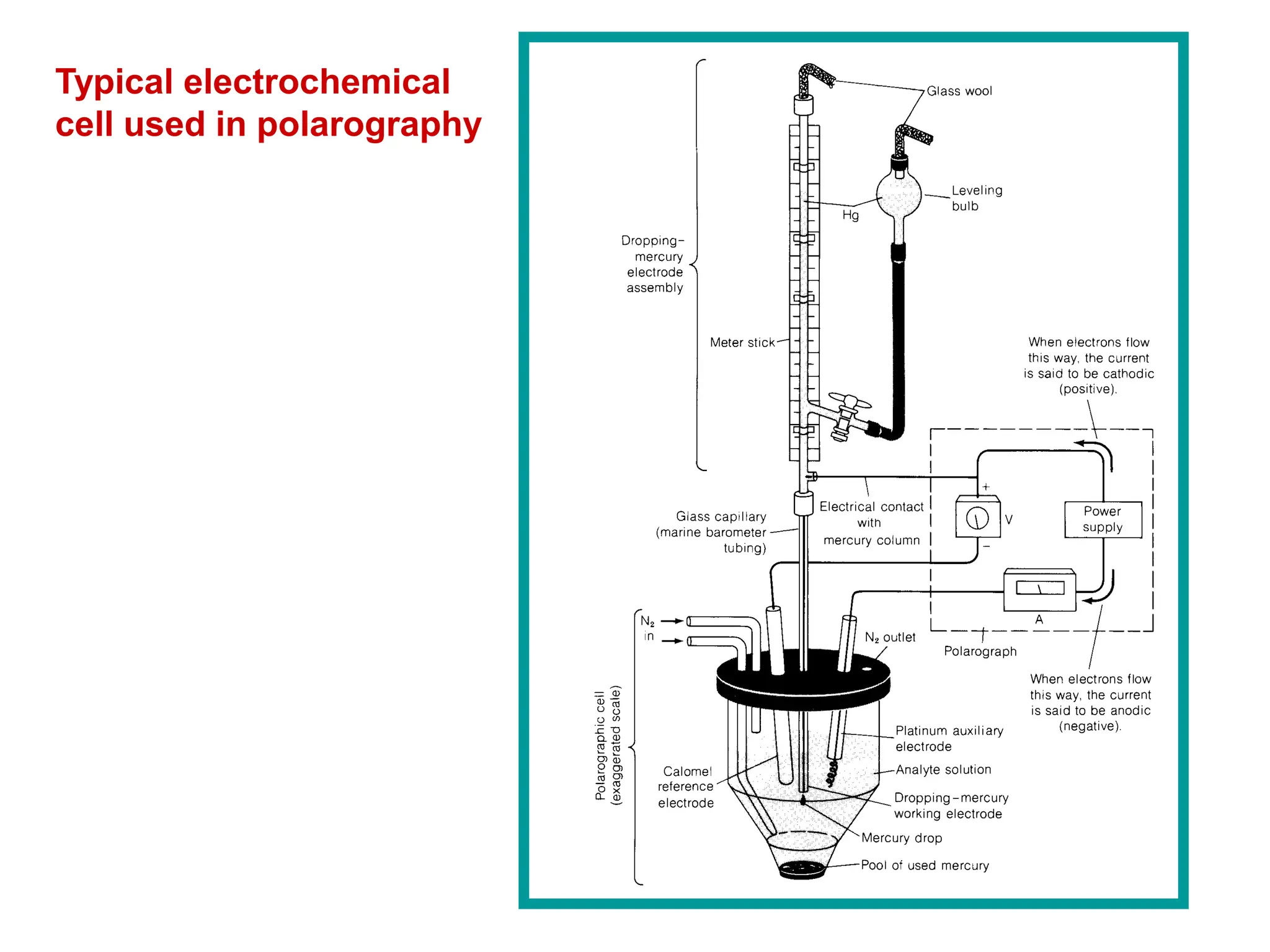
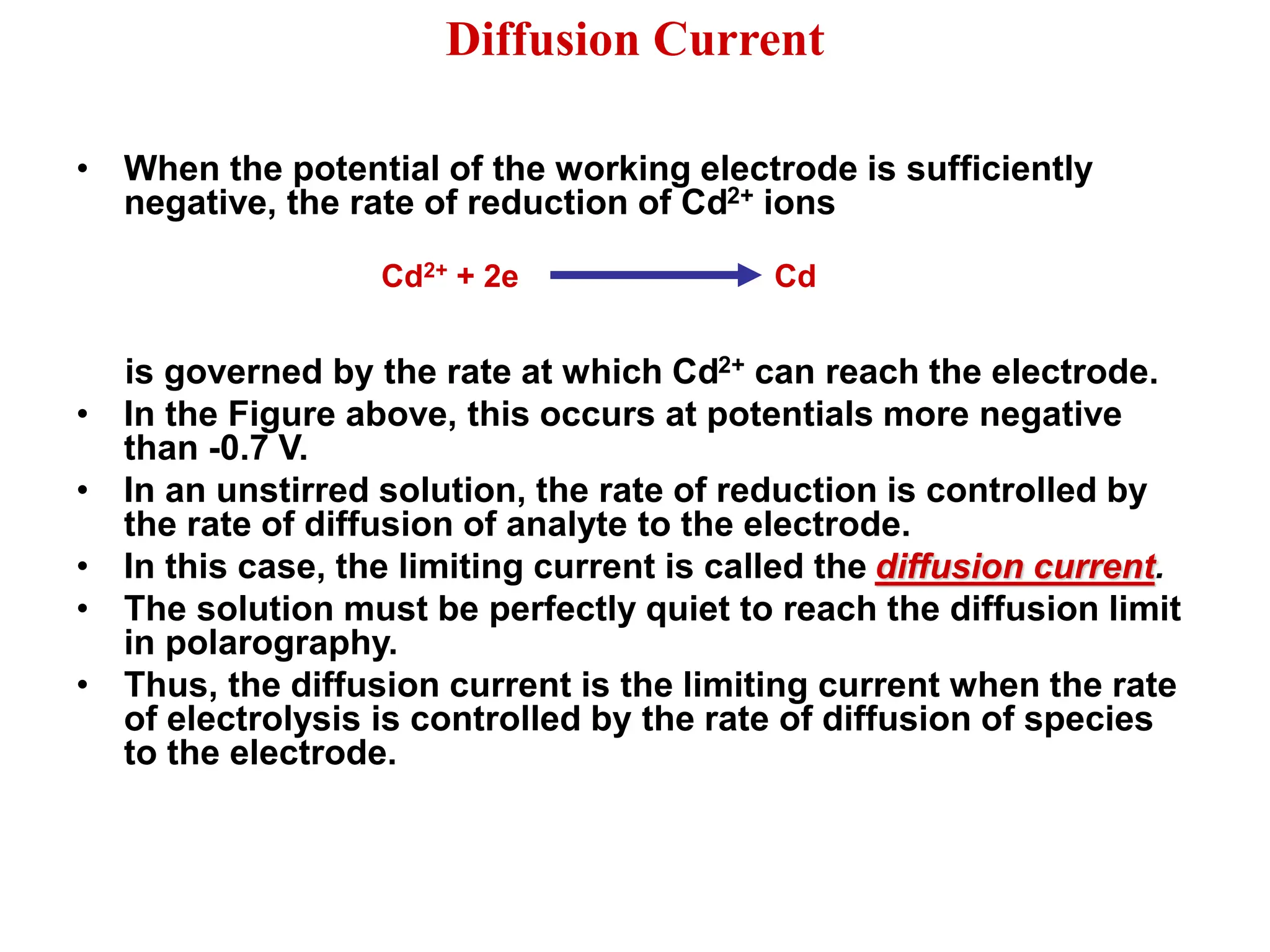
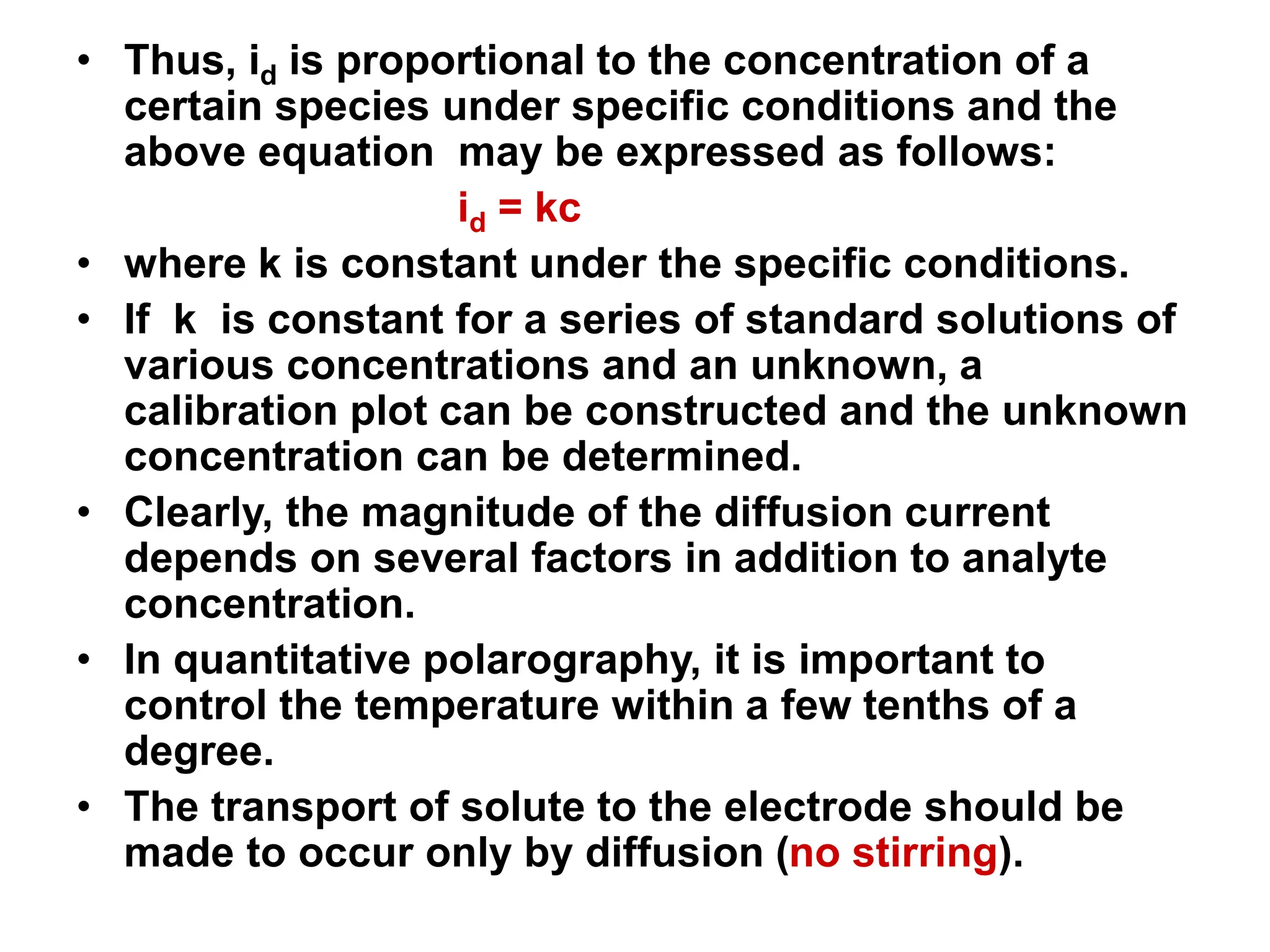
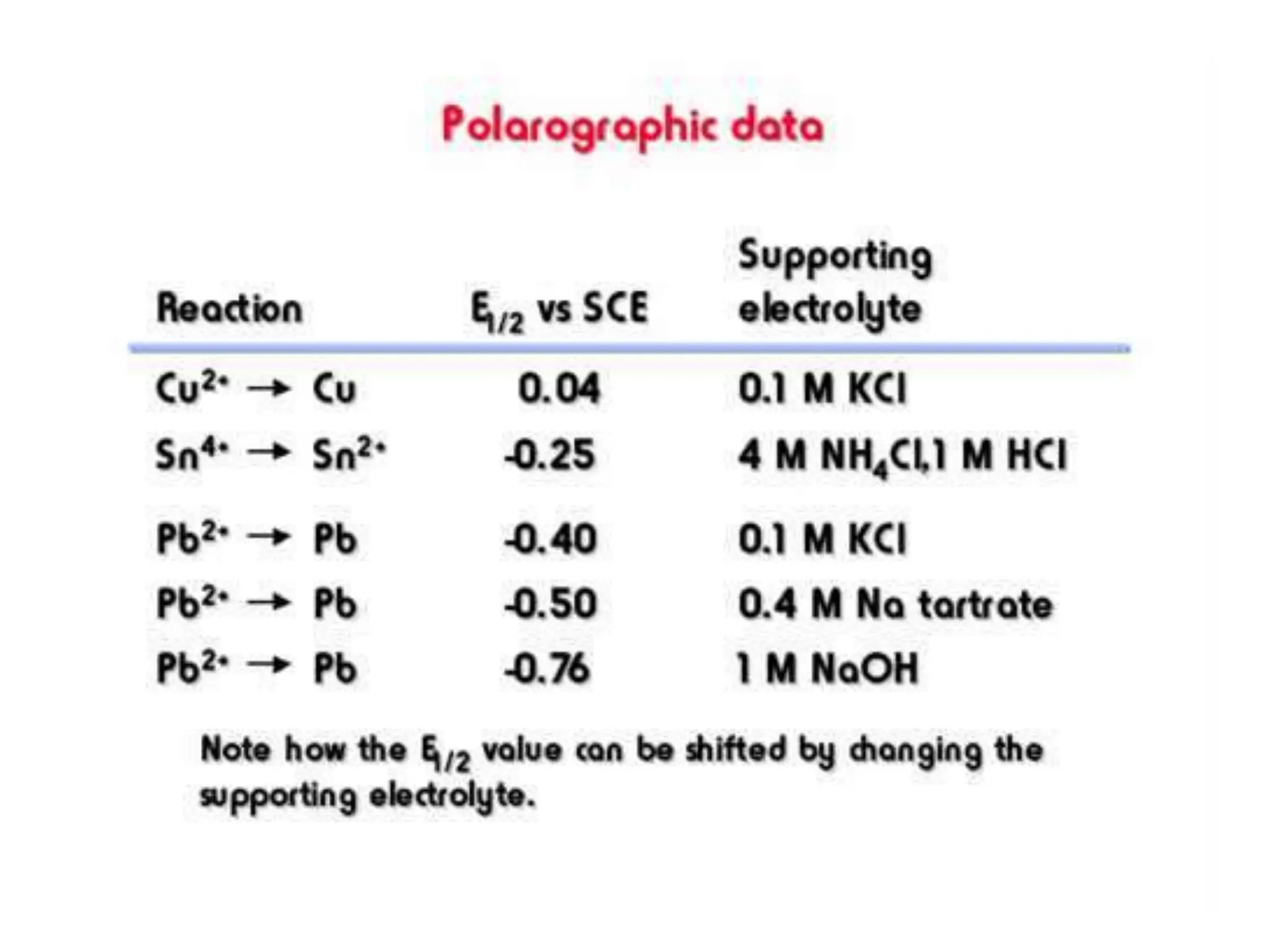
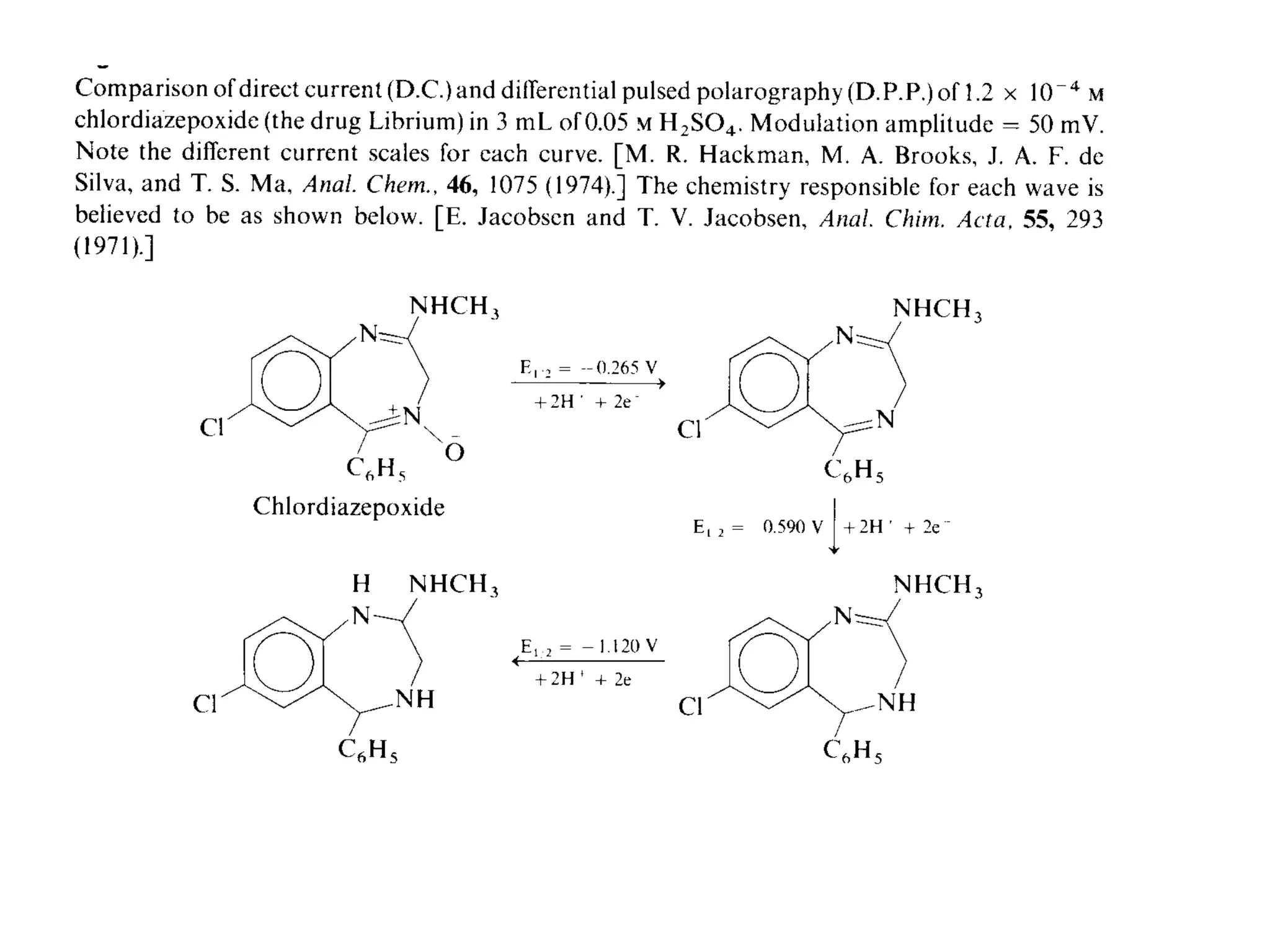
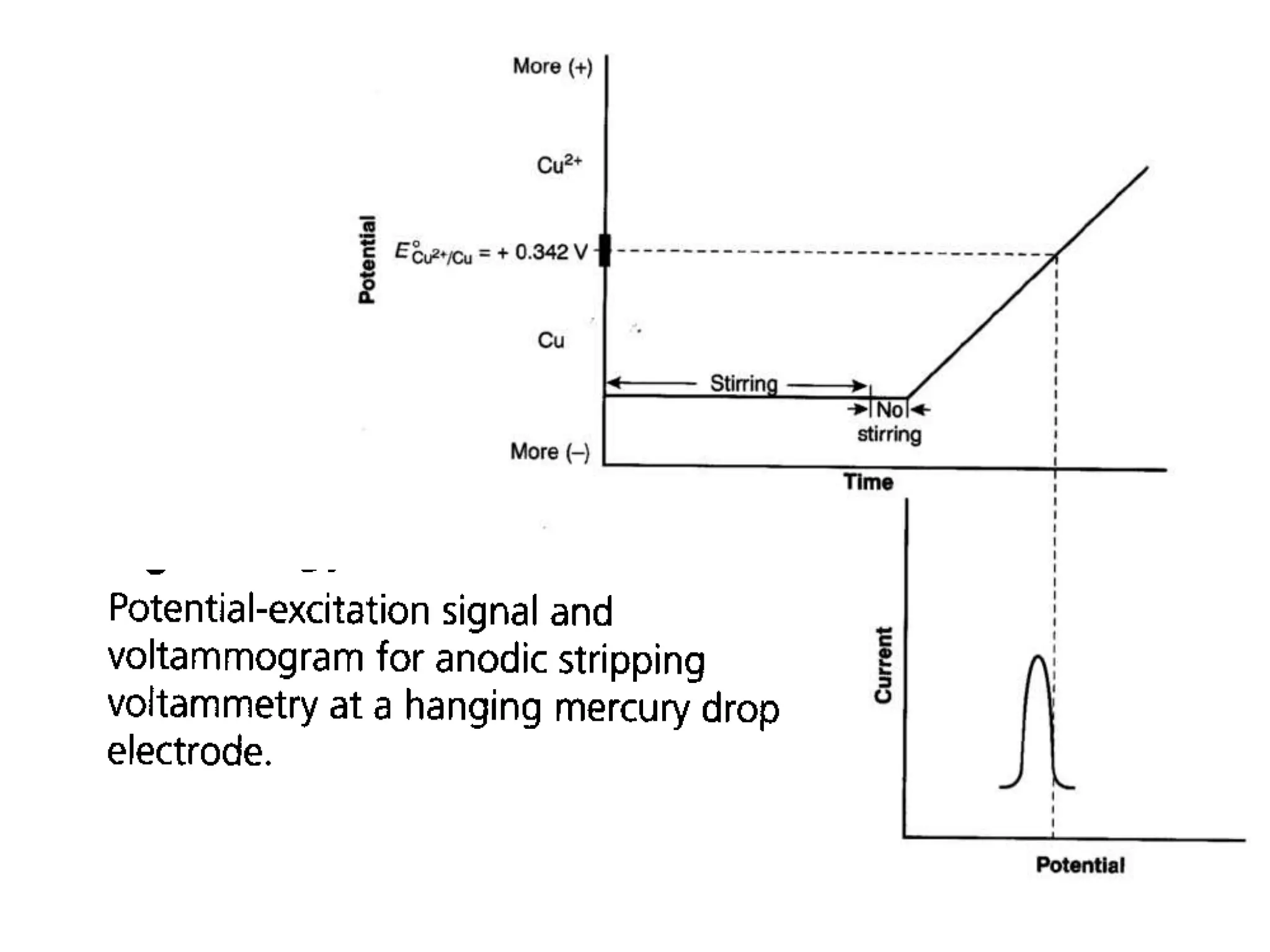
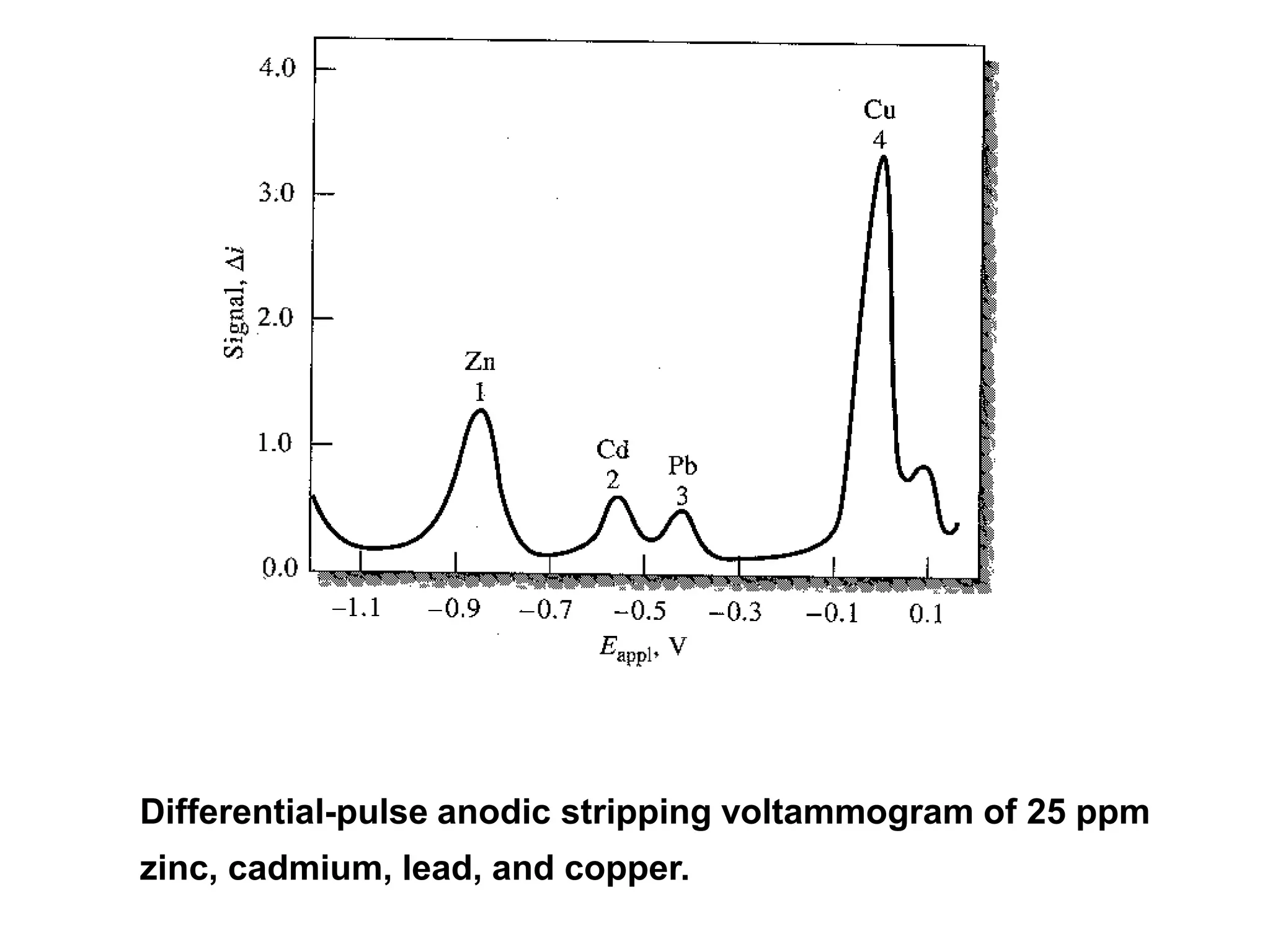
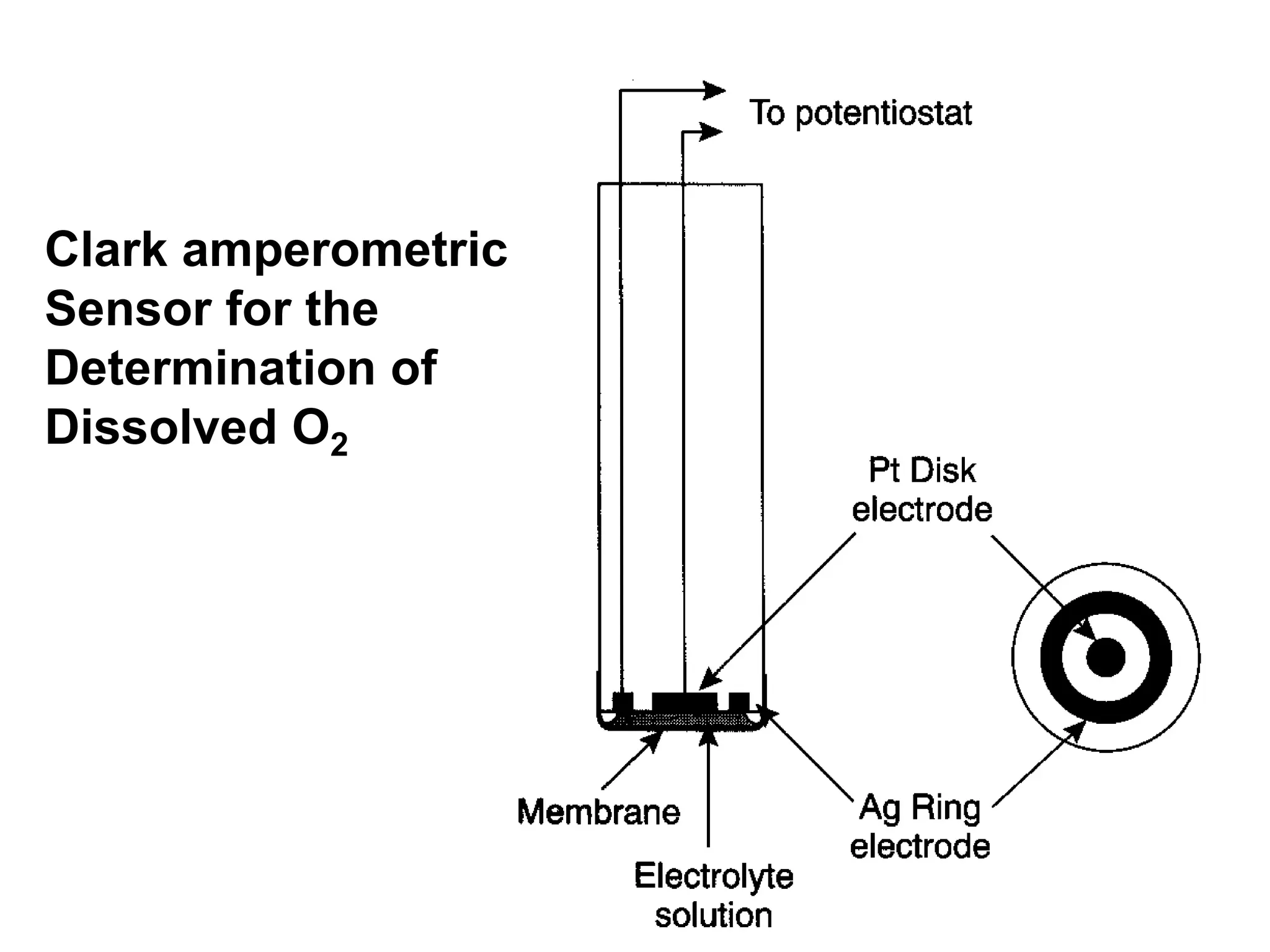
Voltammetry involves applying a time-dependent potential to an electrochemical cell and measuring the resulting current. A voltammogram plots current versus applied potential. Polarography is a type of voltammetry that uses a dropping mercury electrode (DME). With a DME, the potential is measured versus a reference electrode as the mercury drop grows. Current results from redox reactions and is influenced by mass transport and electron transfer kinetics. Non-faradaic currents also occur due to charging of the electrical double layer. A polarogram shows the characteristic diffusion-limited current when sufficient overpotential is applied for an analyte's reduction.



















![• Current rate of diffusion [C]o - [C]s
The [C]o and [C]s are the concentrations in
the bulk solution and at the electrode
surface.
• The greater the difference in concentrations
the more rapid will be the diffusion.
• At a sufficiently negative potential, the
reduction is so fast that the [C]s << [C]o and
equation above reduces to the form
• Limiting current = diffusion current [C]o
• The ratio of the diffusion current to the bulk
solute concentration is the basis for the use
of voltammetry in analytical chemistry](https://image.slidesharecdn.com/4214730-240413051014-acdd9866/75/4214730-voltammetry-and-polarography-ppt-25-2048.jpg)


















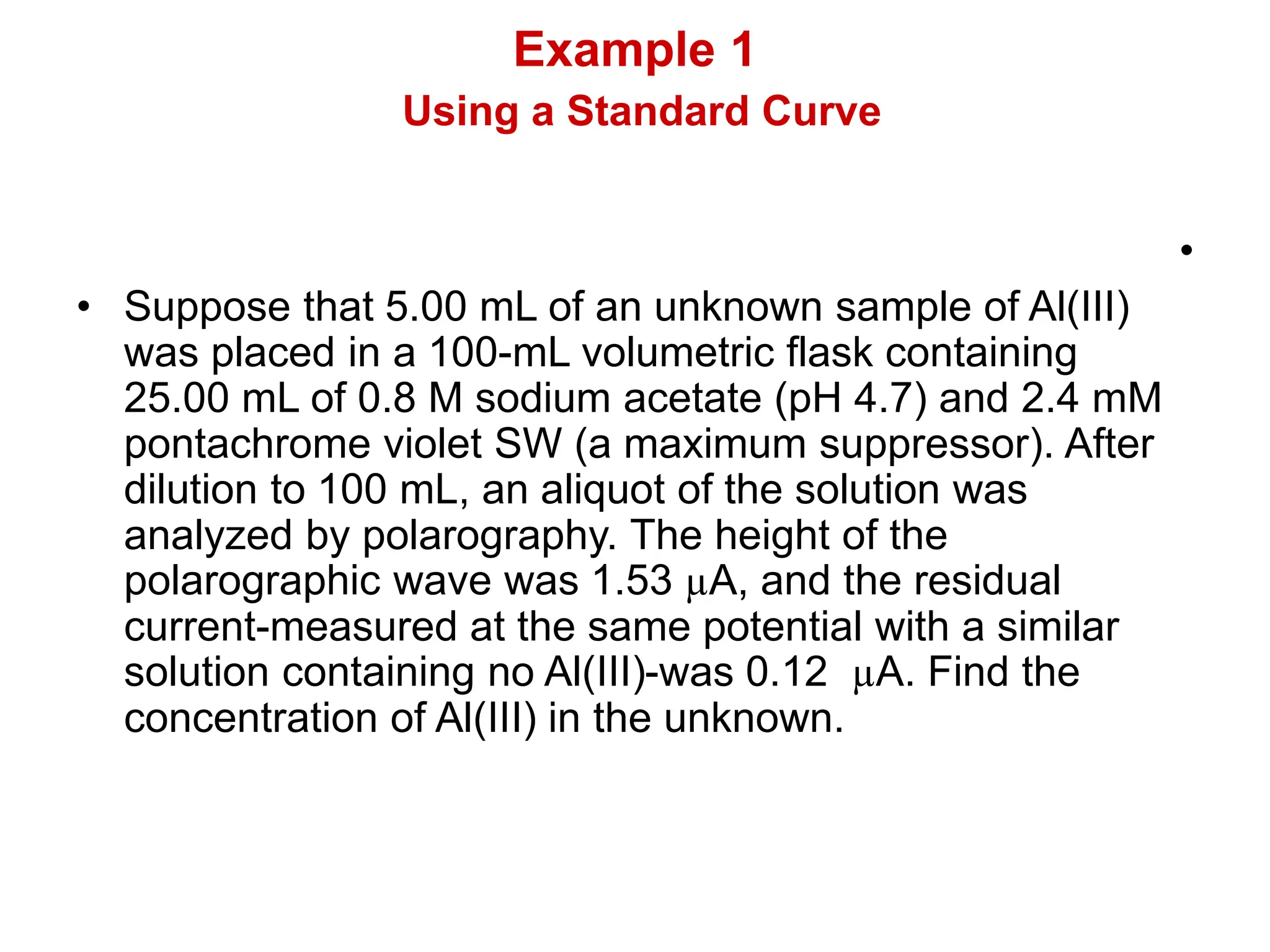
![• The corrected diffusion current is
1.53 - 0.12 = 1.41 µA.
• In the figure above, 1.41 µA
corresponds to [AI(III)] = 0.126 mm.
• Since the unknown was diluted by a
factor of 20.0 (from 5.00 mL to 100 mL)
for analysis, the original concentration
of unknown must have been
(20.0)(0.126) = 2.46 mm.](https://image.slidesharecdn.com/4214730-240413051014-acdd9866/75/4214730-voltammetry-and-polarography-ppt-51-2048.jpg)