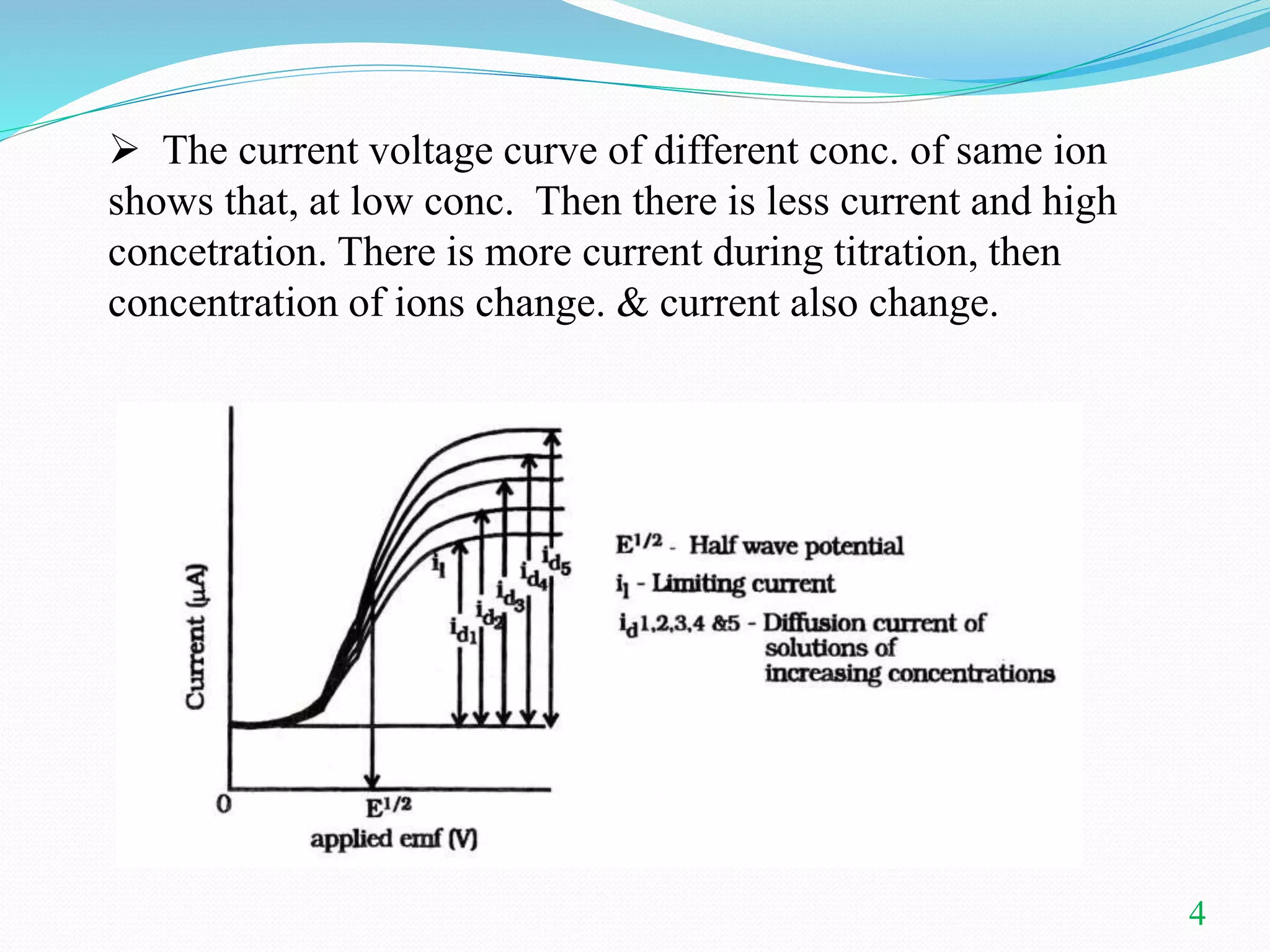
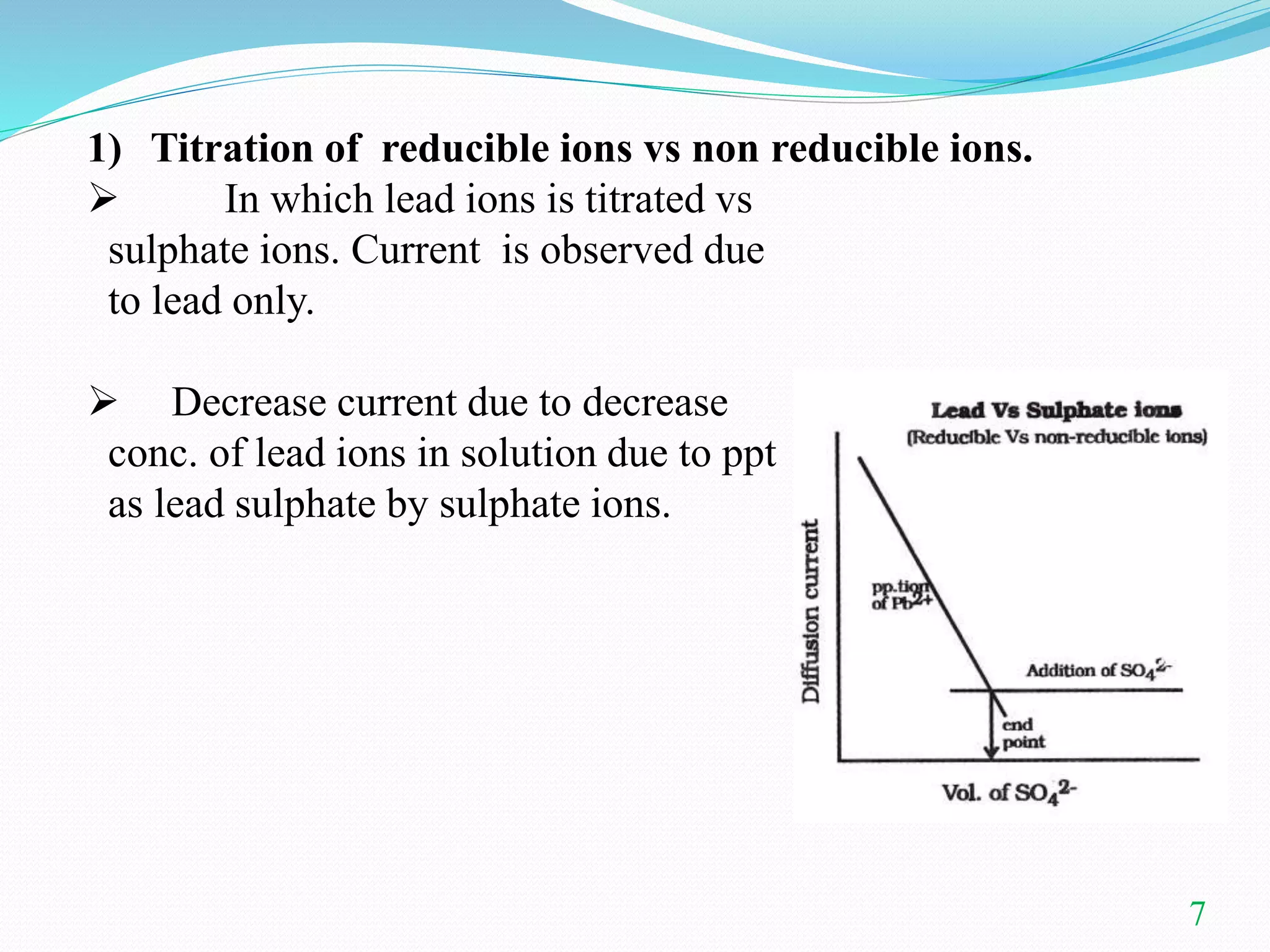
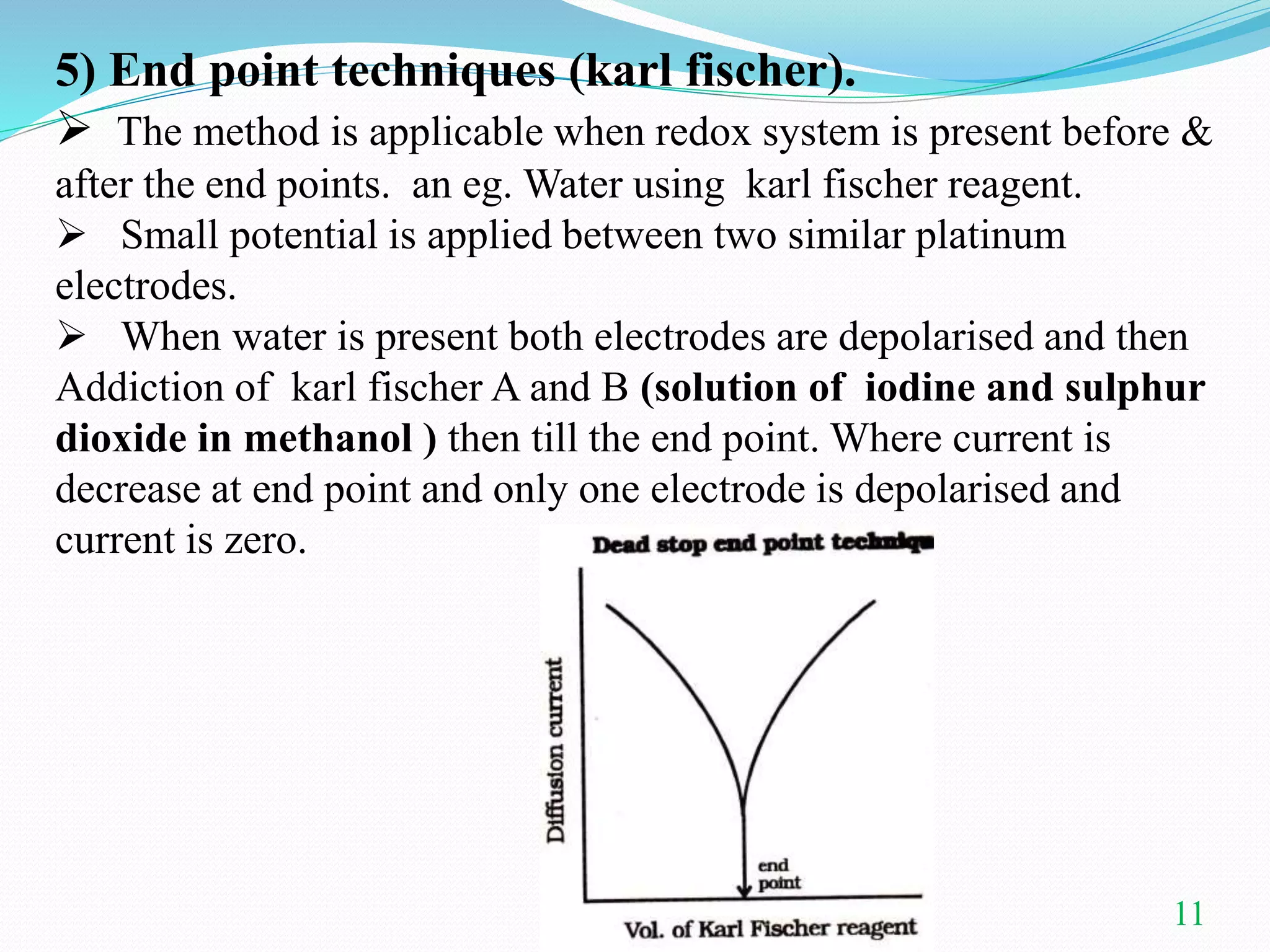
This document summarizes amperometric titration, a technique where the current is measured during titration using a constant applied potential. It discusses the principles, apparatus, types (including titration of reducible vs. non-reducible ions and redox titrations), advantages, and applications of amperometric titration. The rotating platinum microelectrode apparatus allows a potential to be applied while measuring the current. Amperometric titration can be used to quantify reducible and non-reducible ions and is useful for dilute solutions.