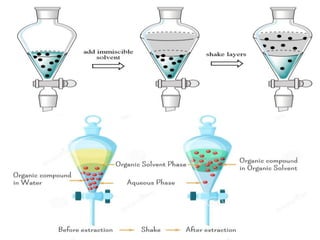
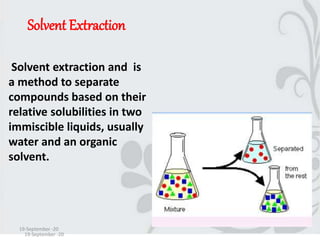
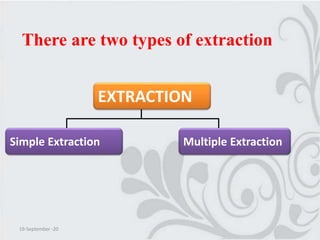
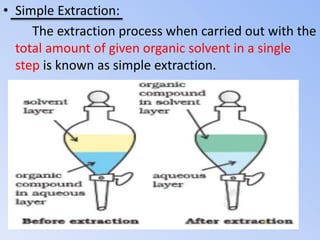
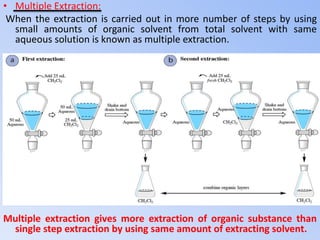
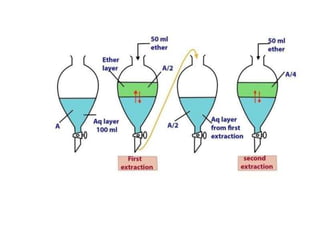
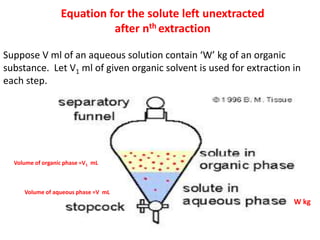
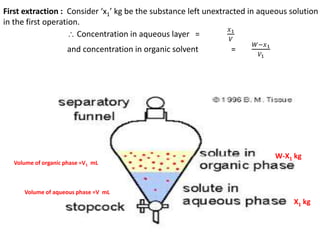
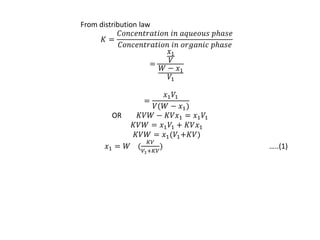
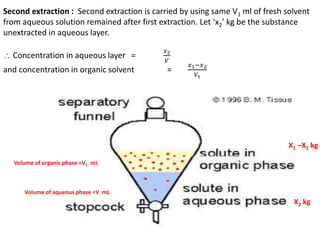
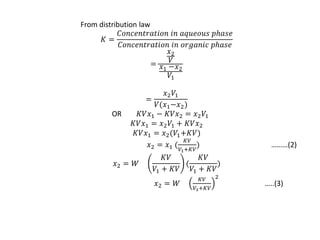
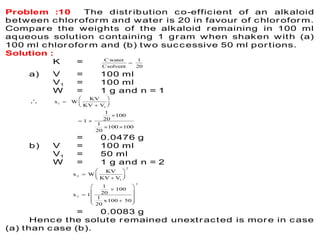
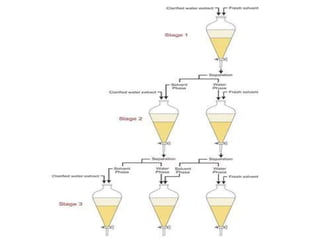
This document discusses solvent extraction, which uses the relative solubilities of compounds in immiscible liquids like water and an organic solvent to separate mixtures. It defines simple extraction as a single step process using the total solvent amount, while multiple extraction uses smaller portions of solvent over multiple steps. An equation is provided to calculate the amount of solute left unextracted after the nth extraction step based on the distribution coefficient K, volumes of solvents V and V1, and initial solute amount W. Two example problems demonstrate using this equation to determine extraction amounts for given conditions.