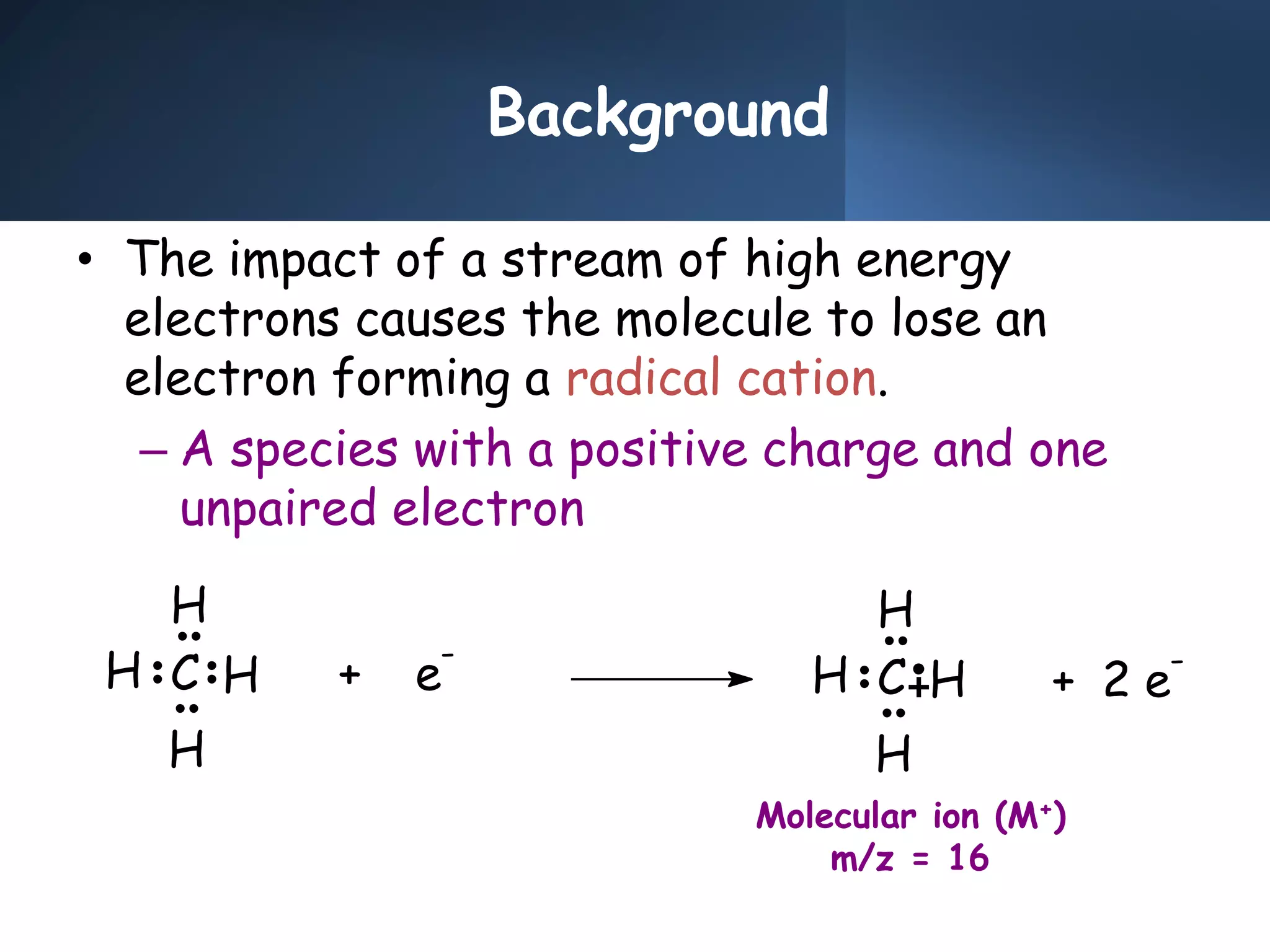
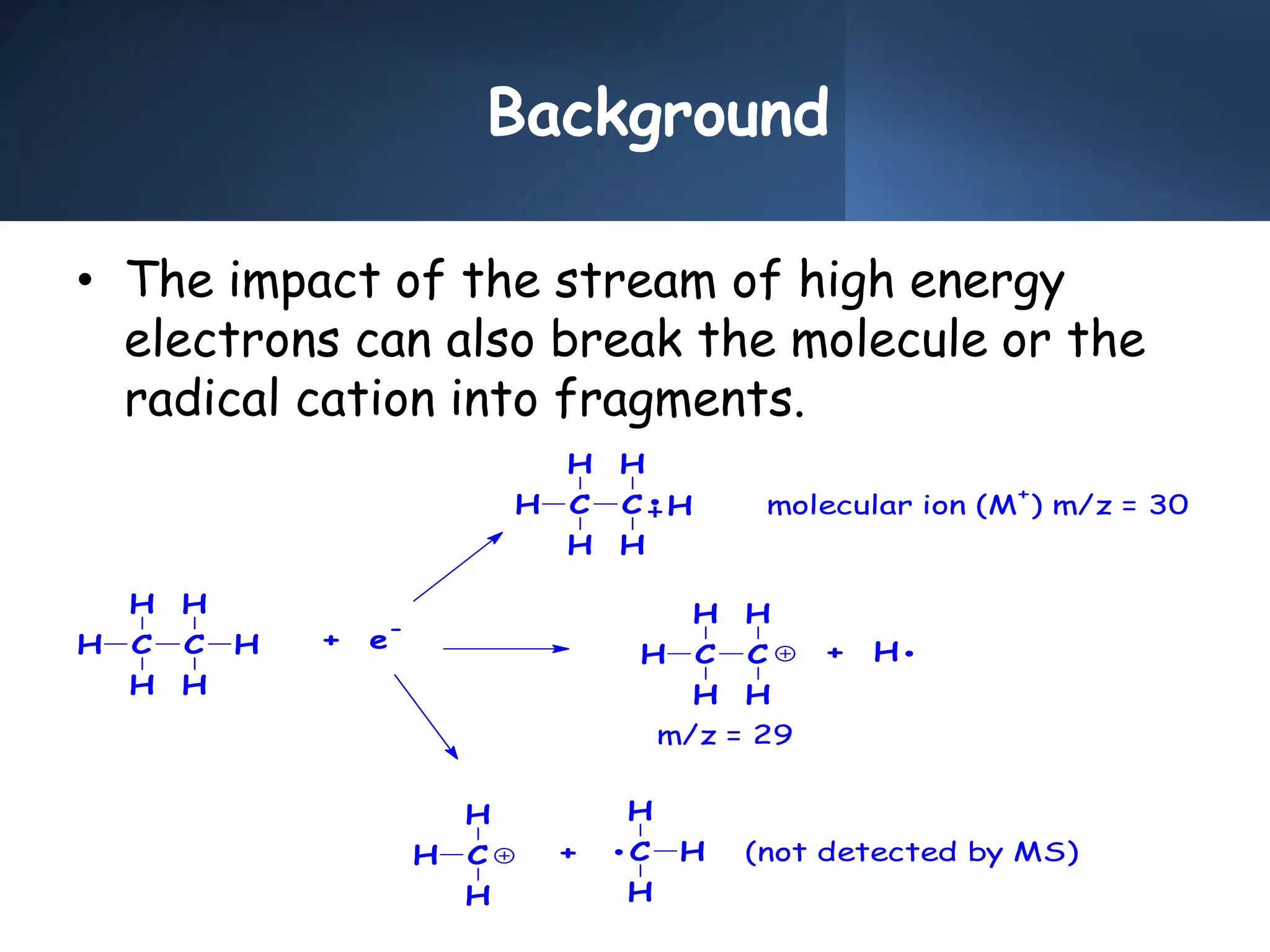
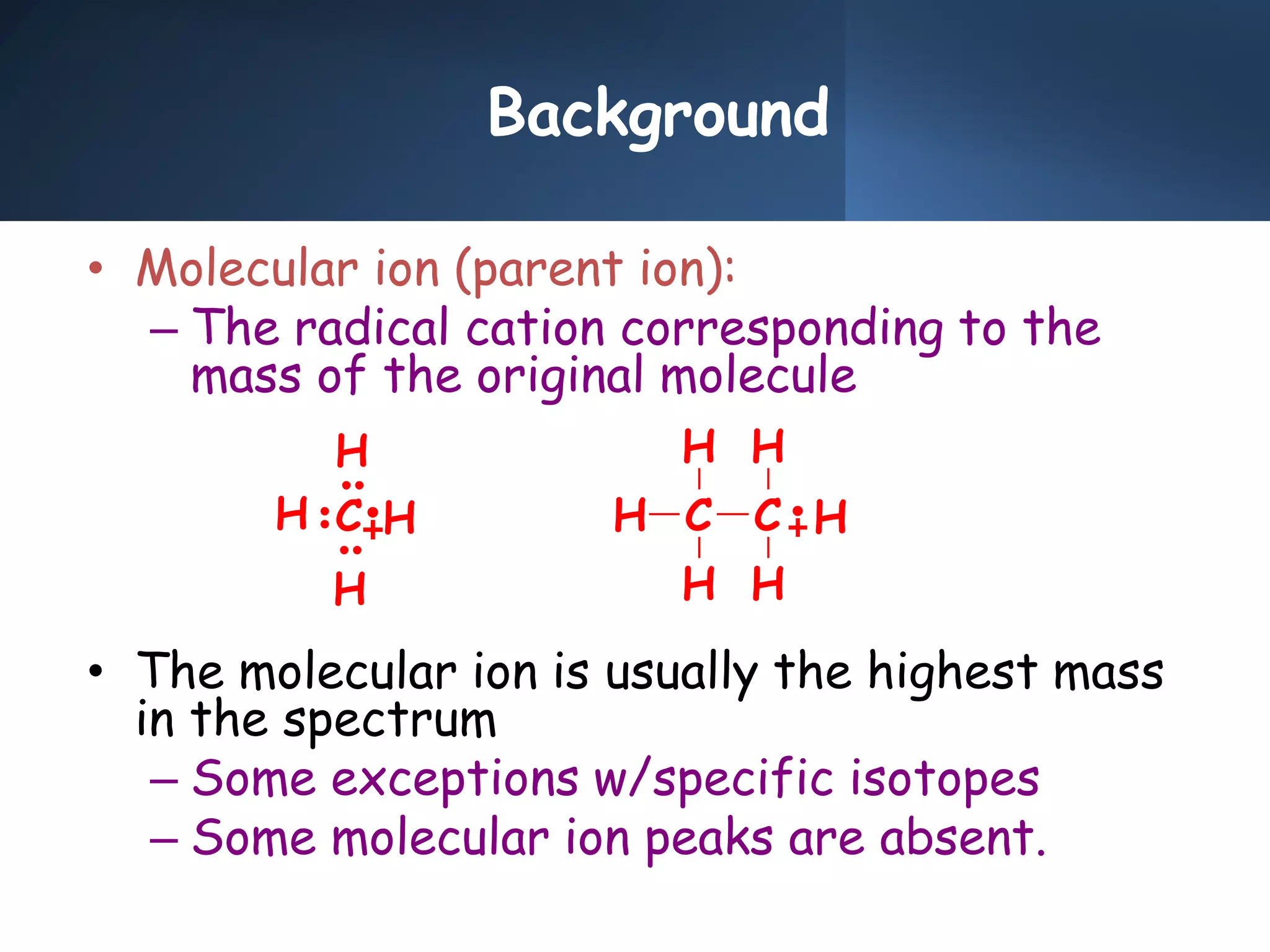
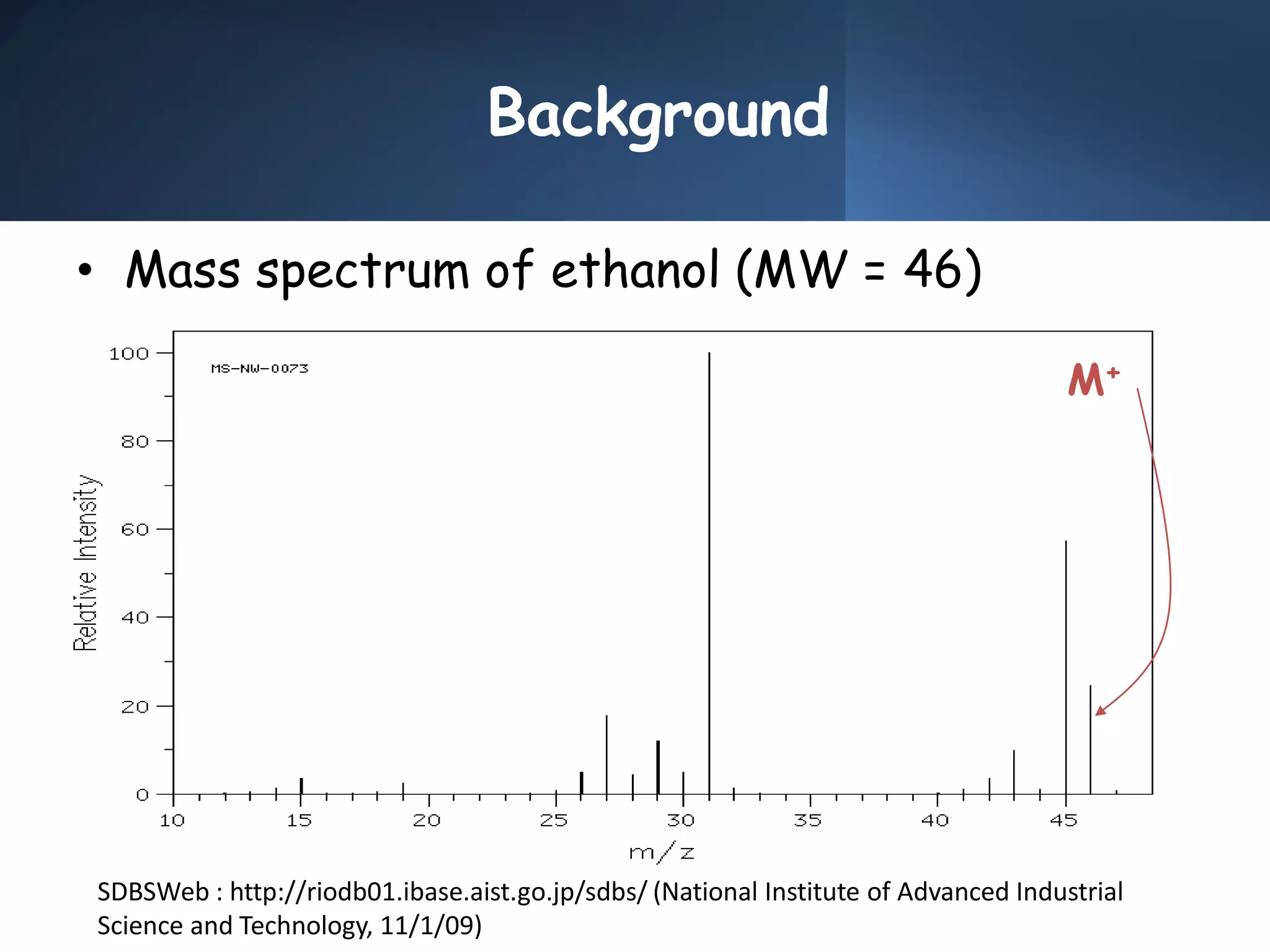
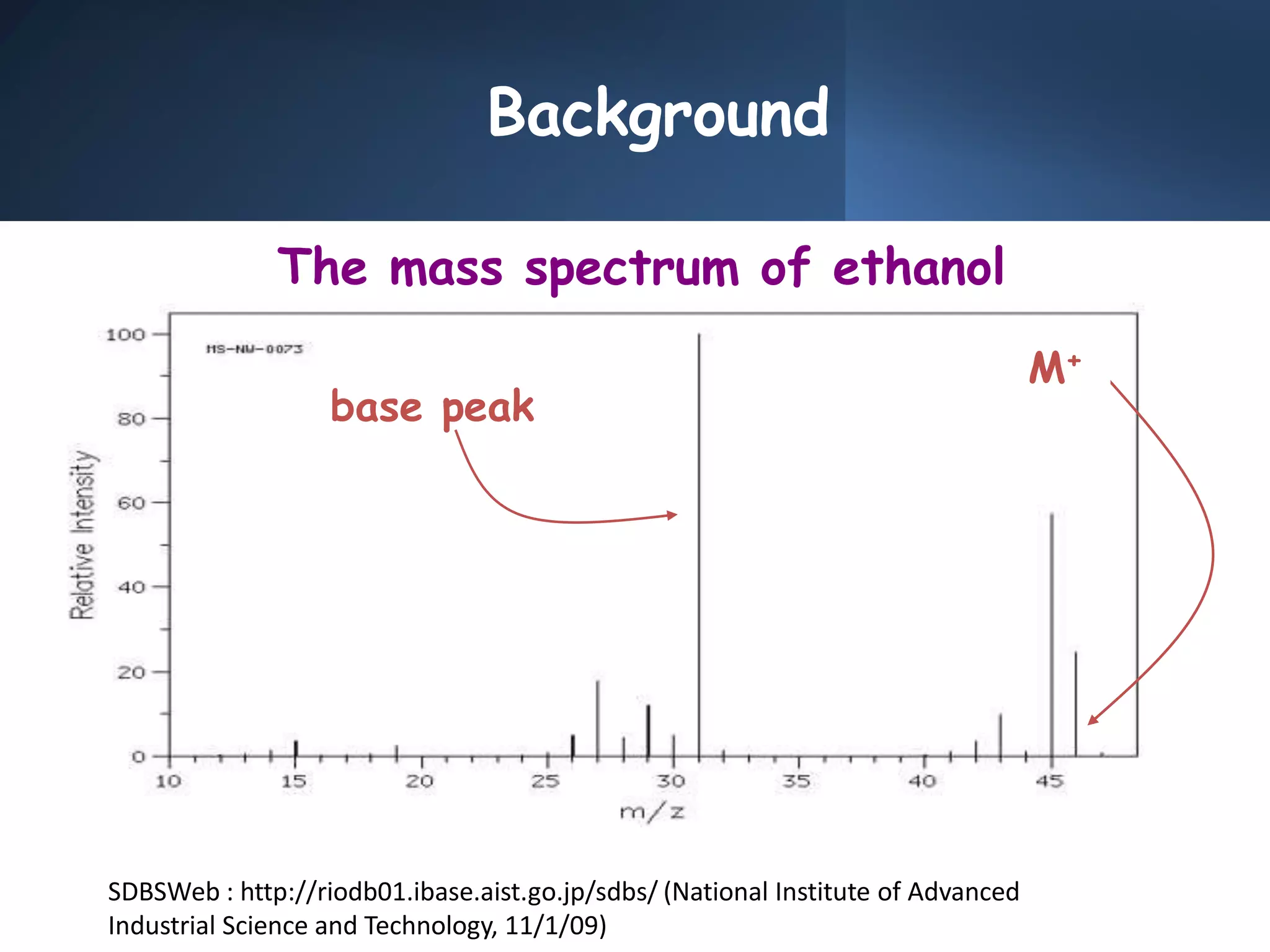
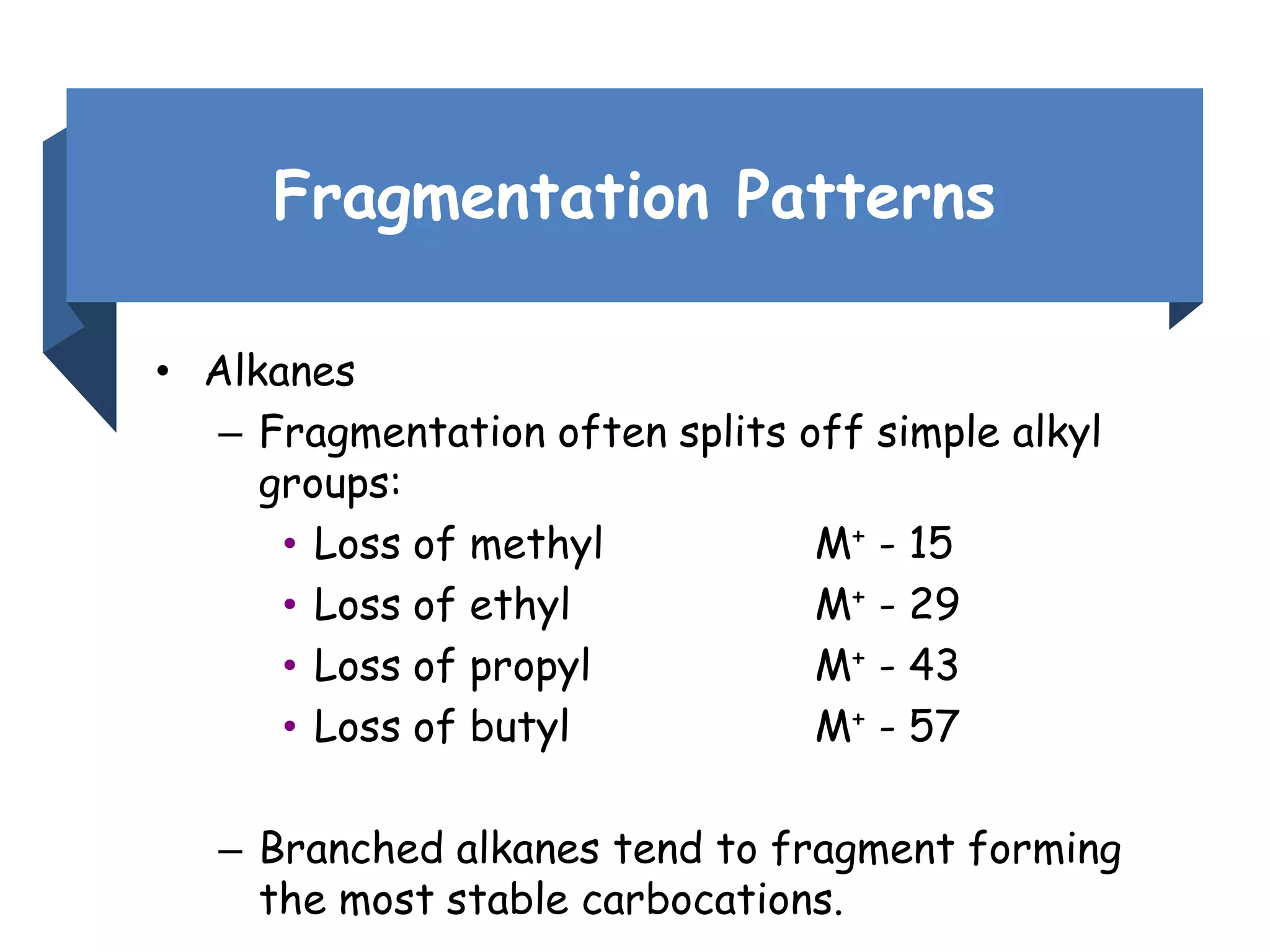
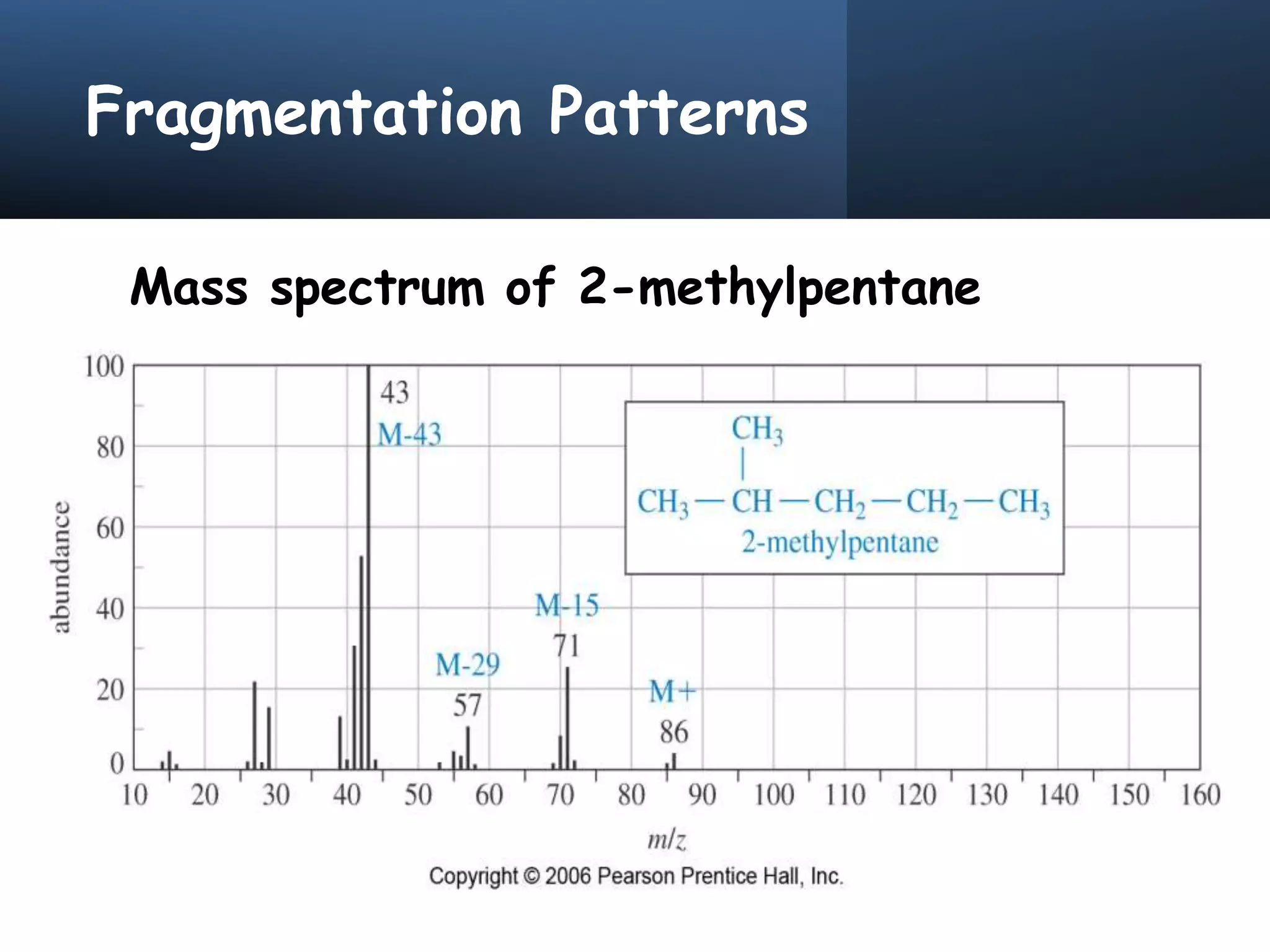
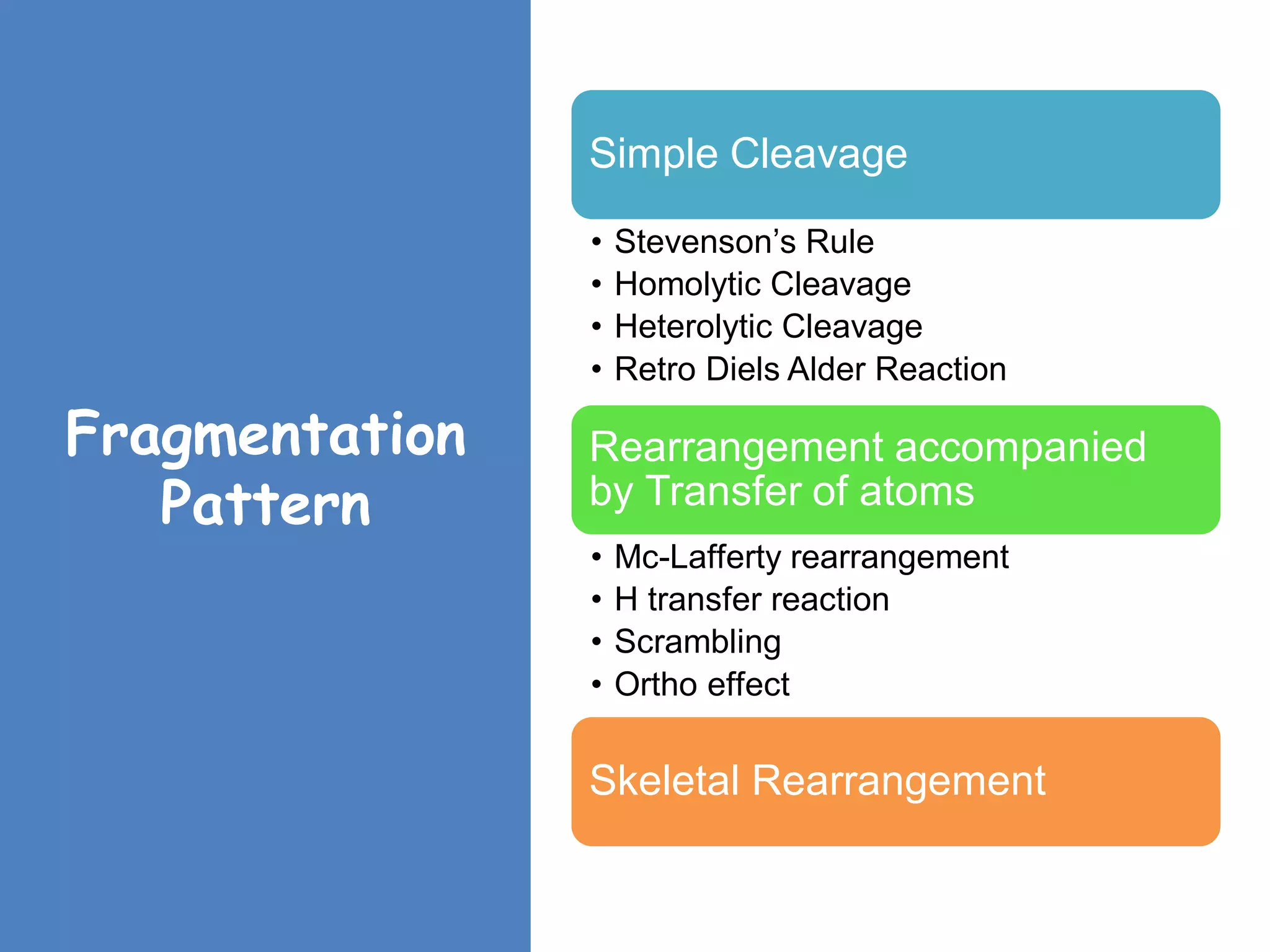
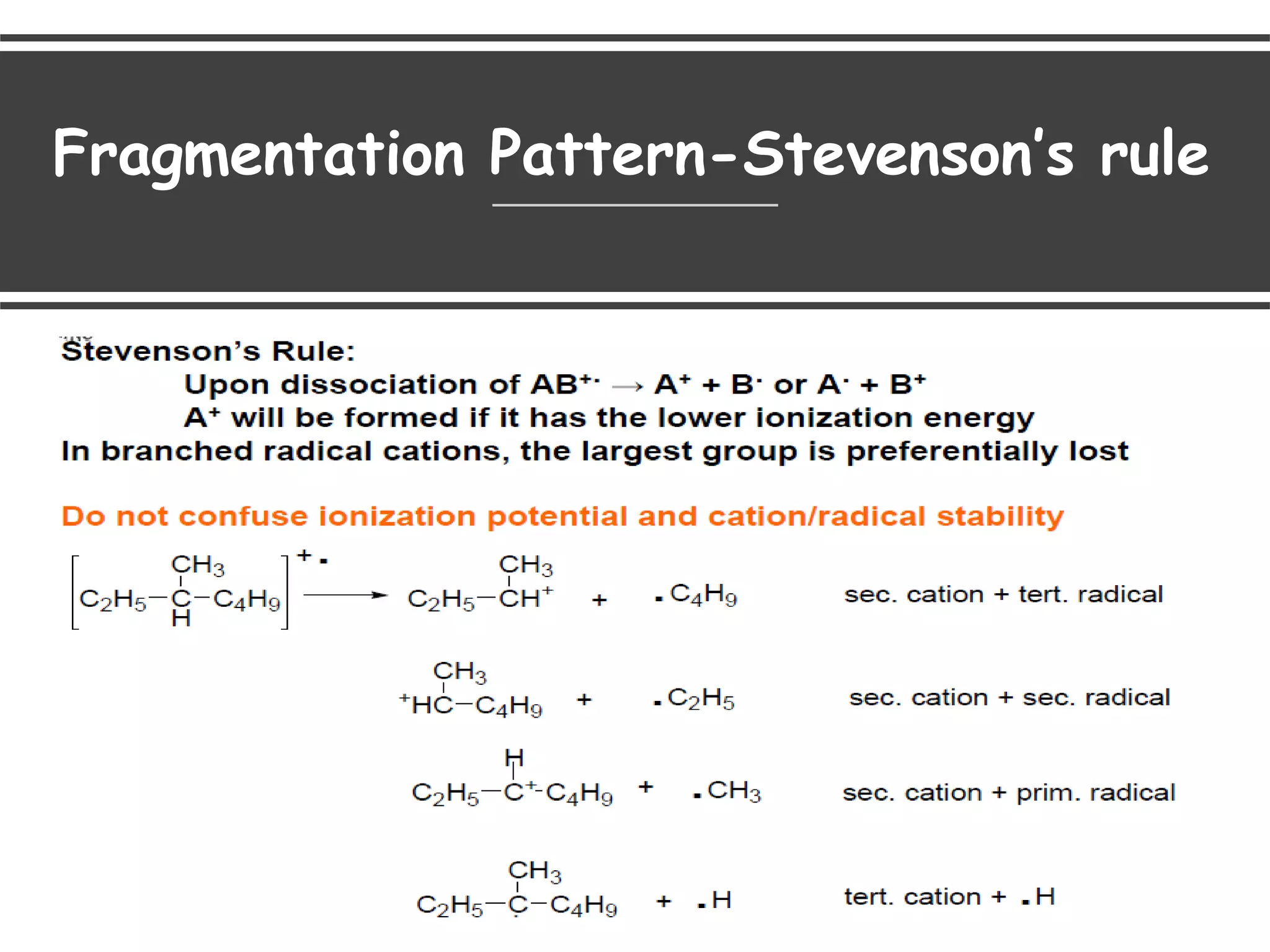
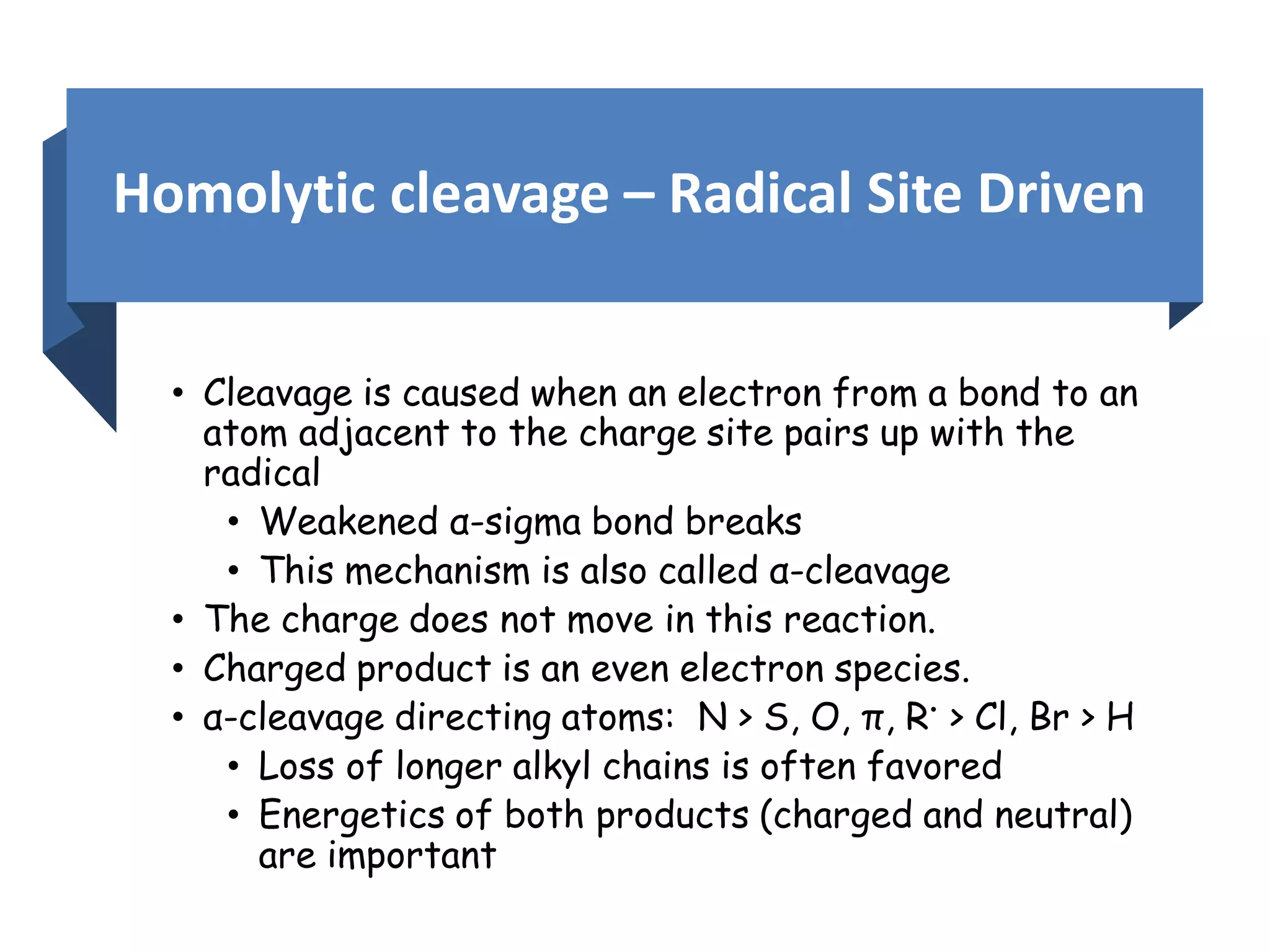
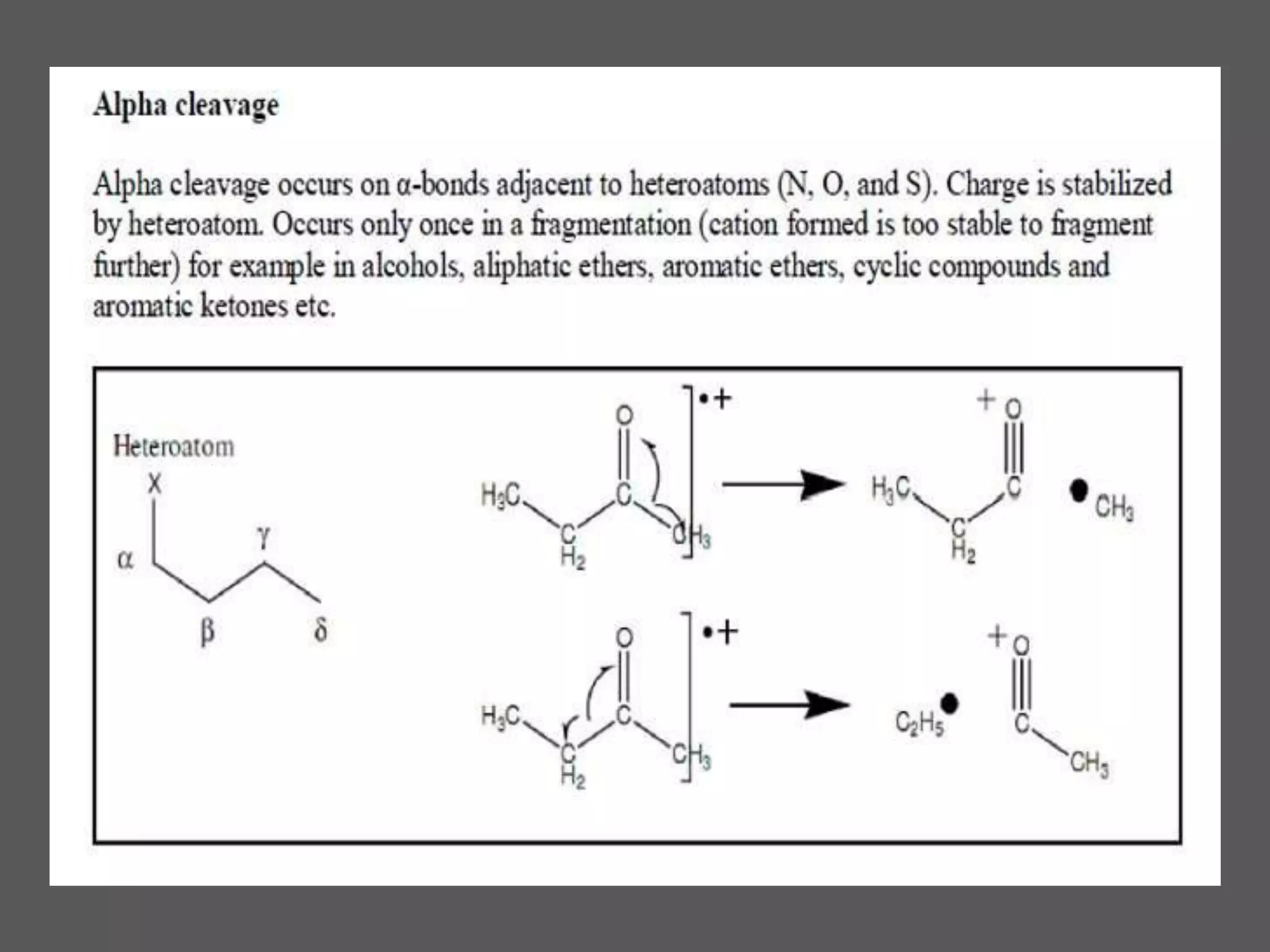
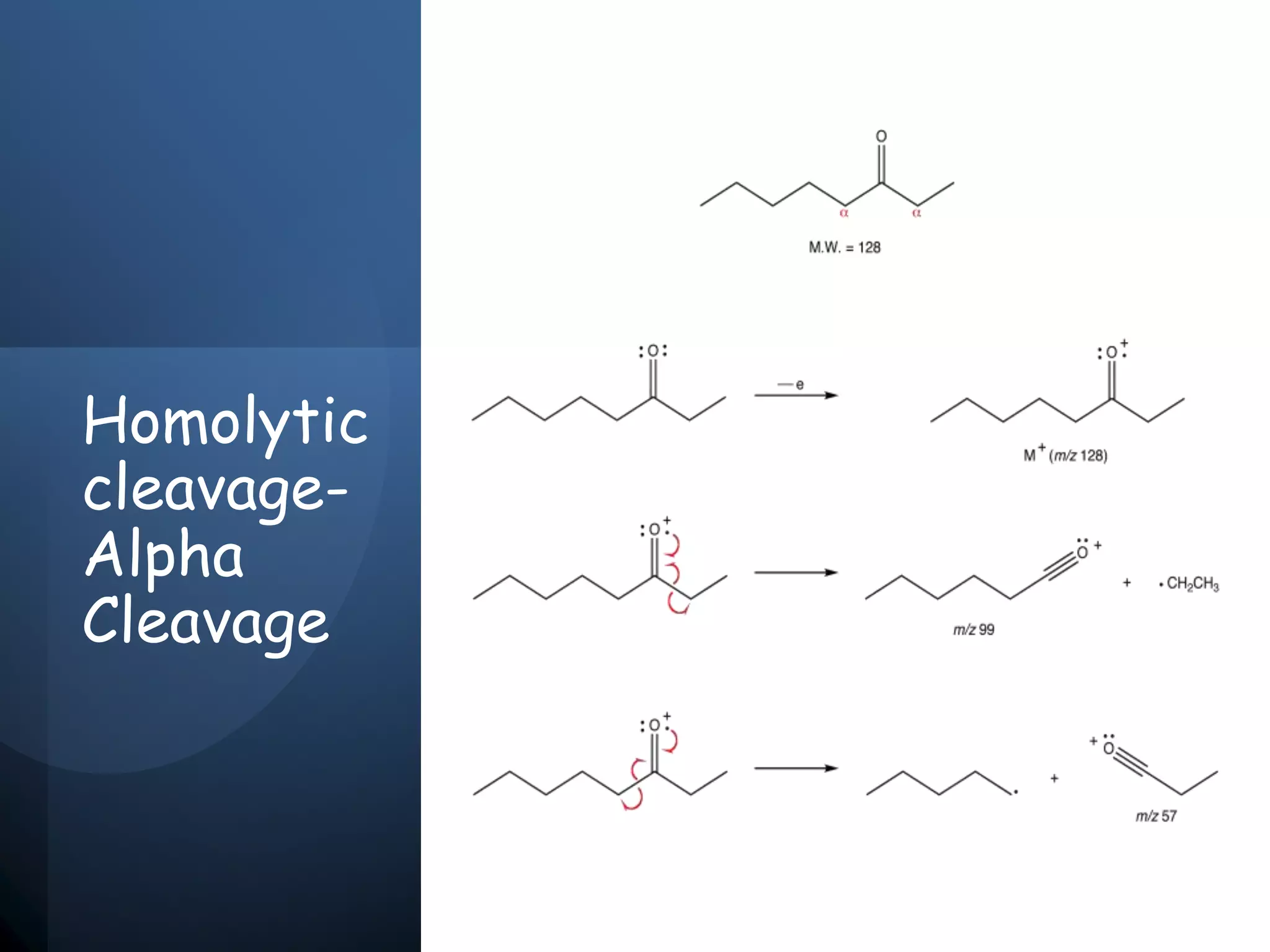
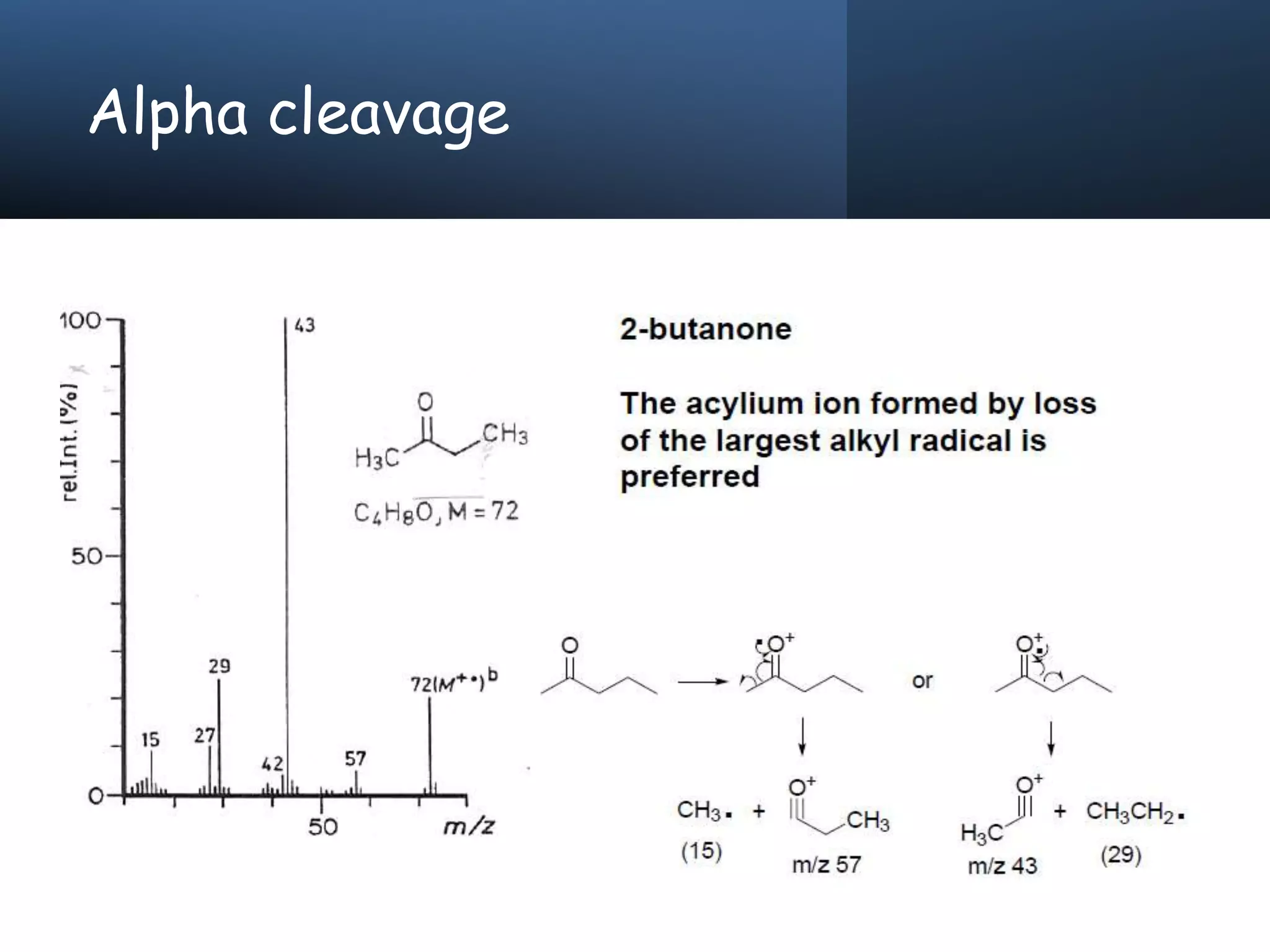
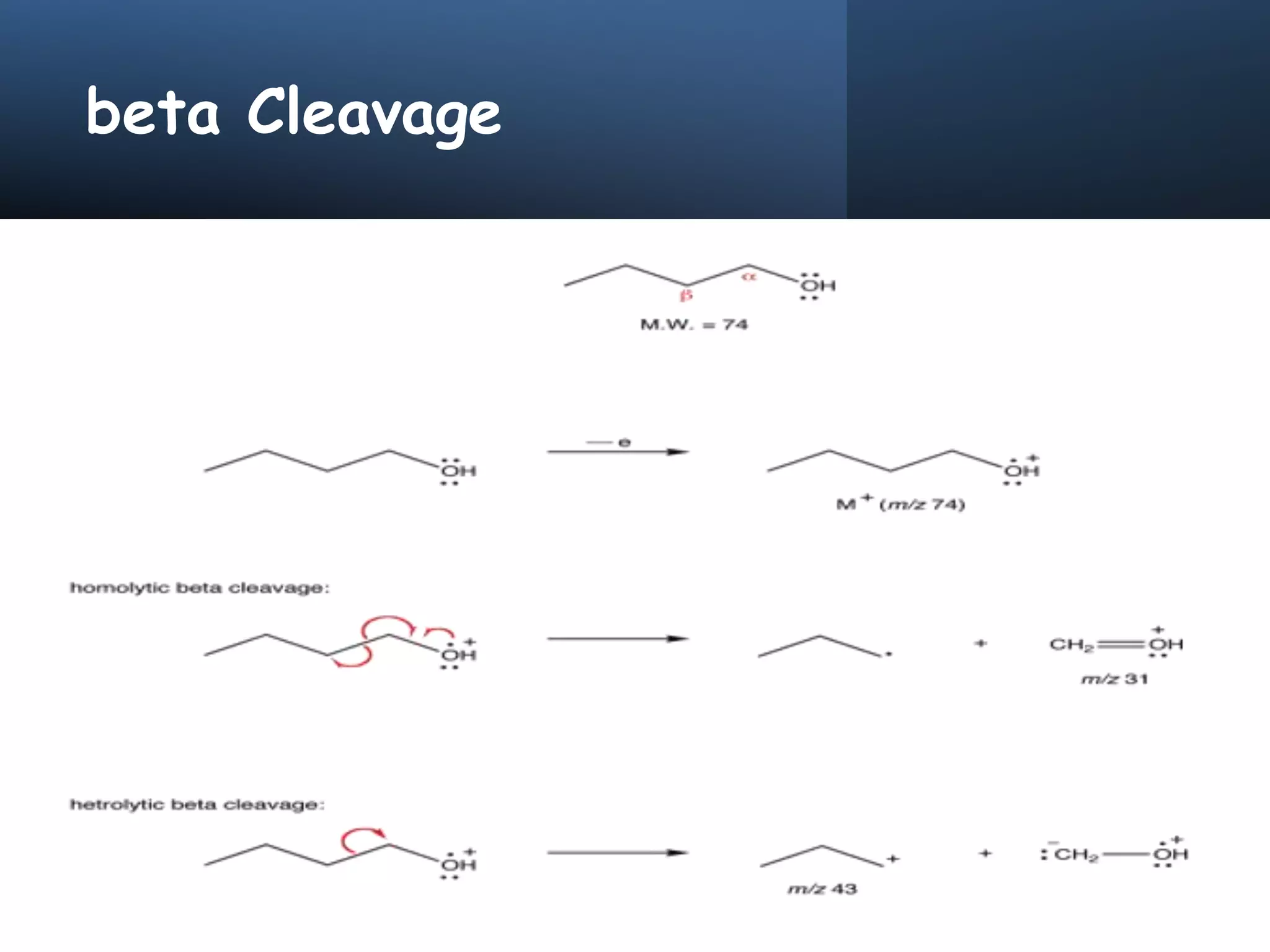
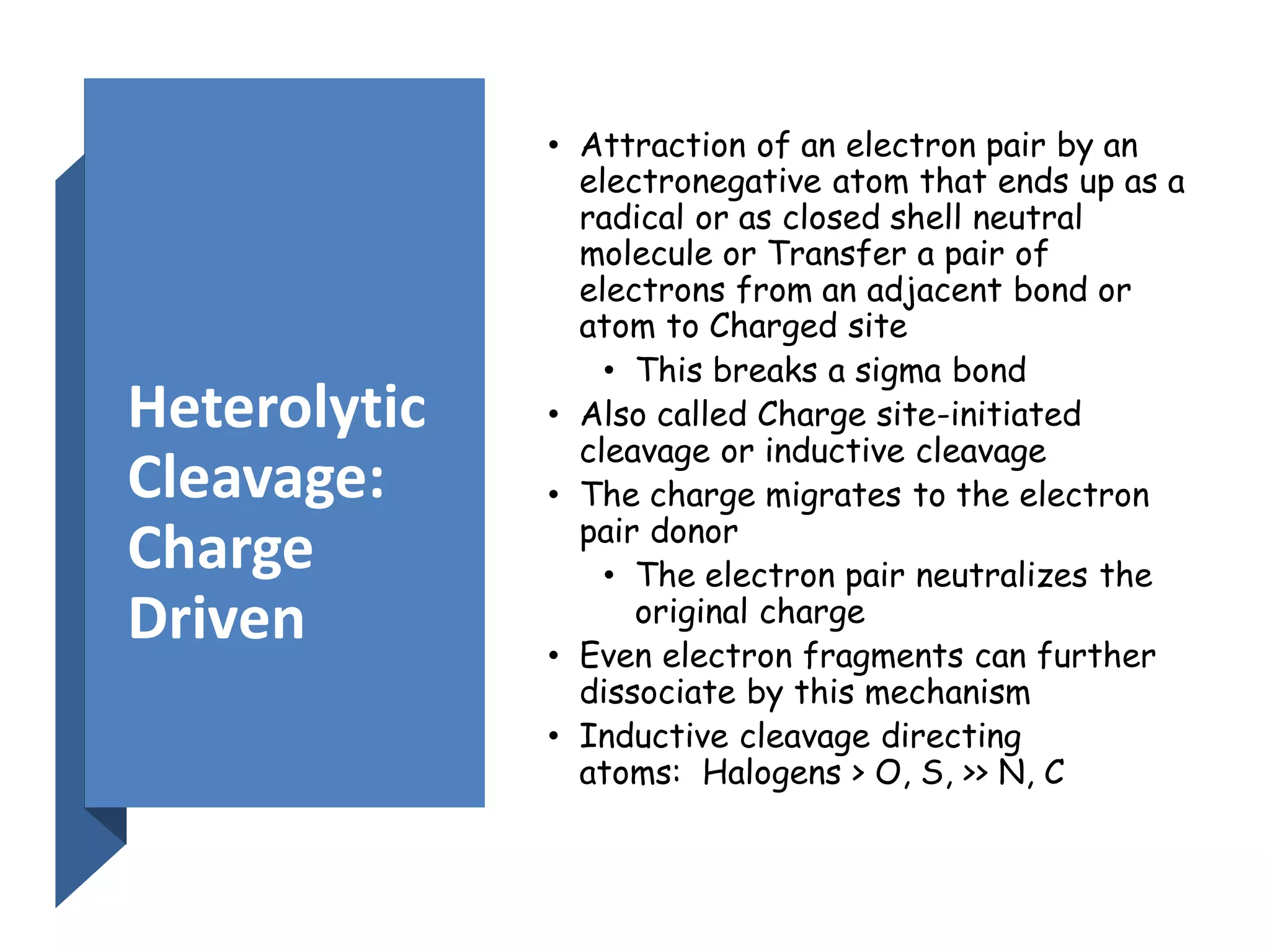
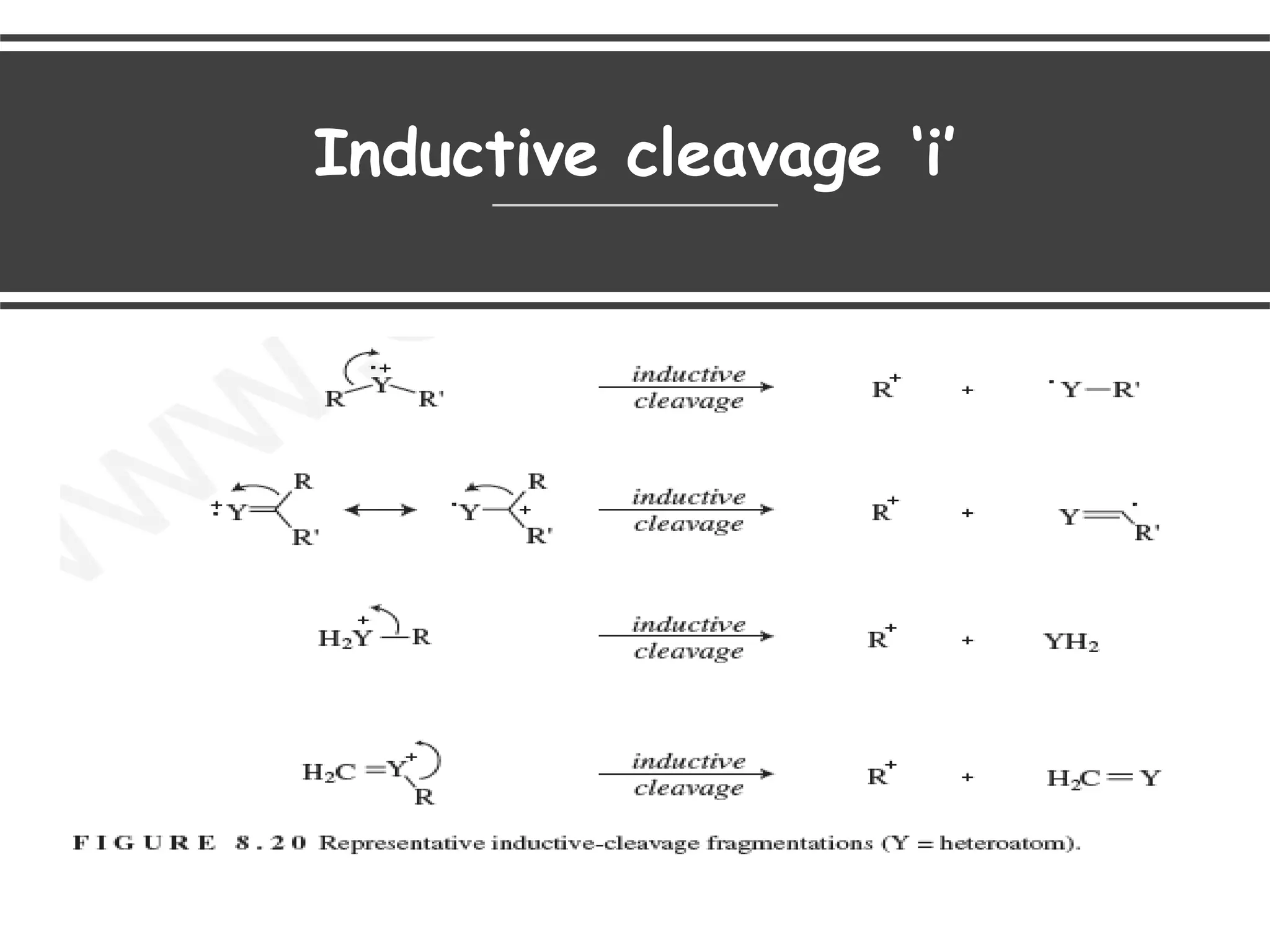
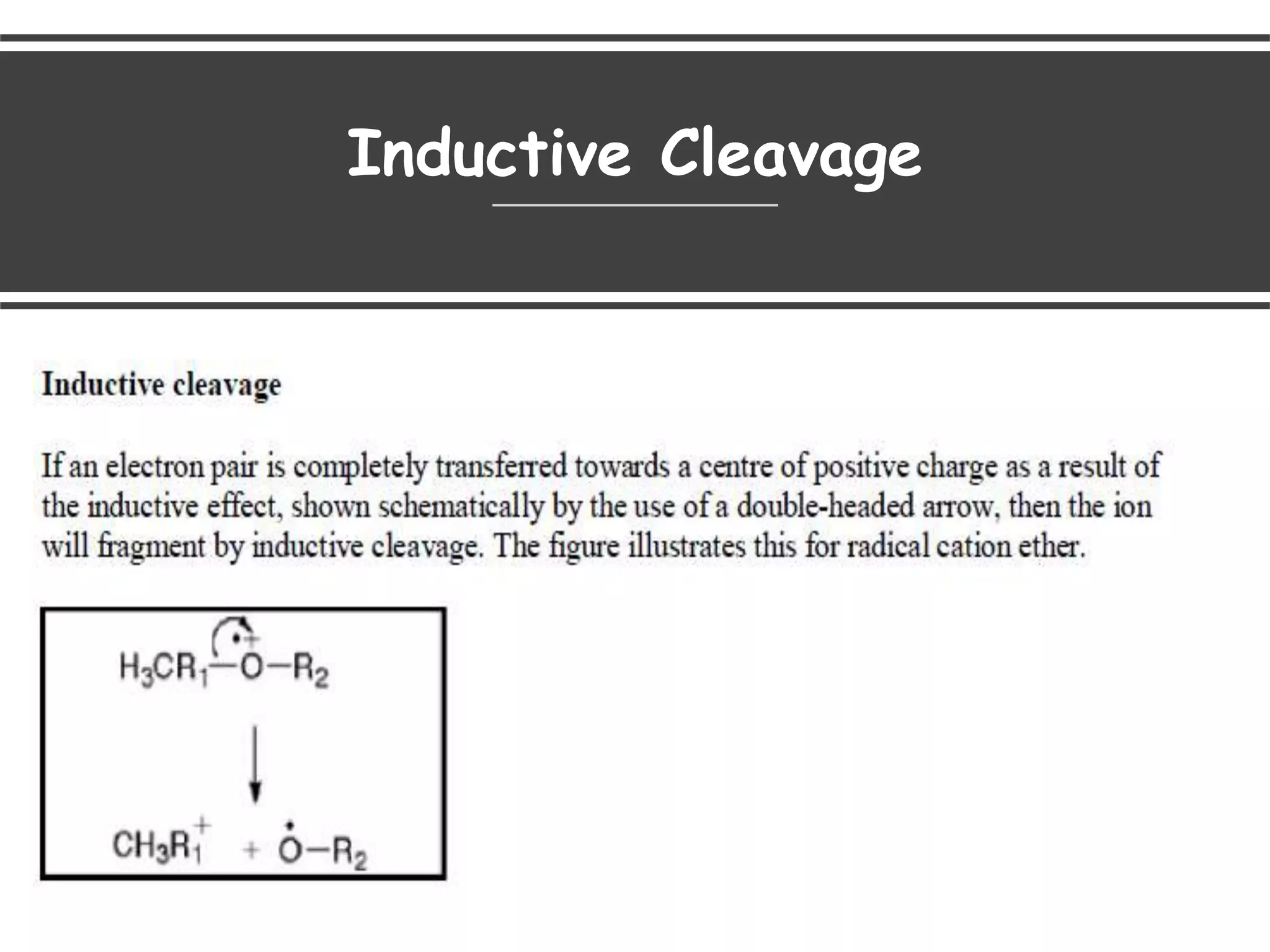
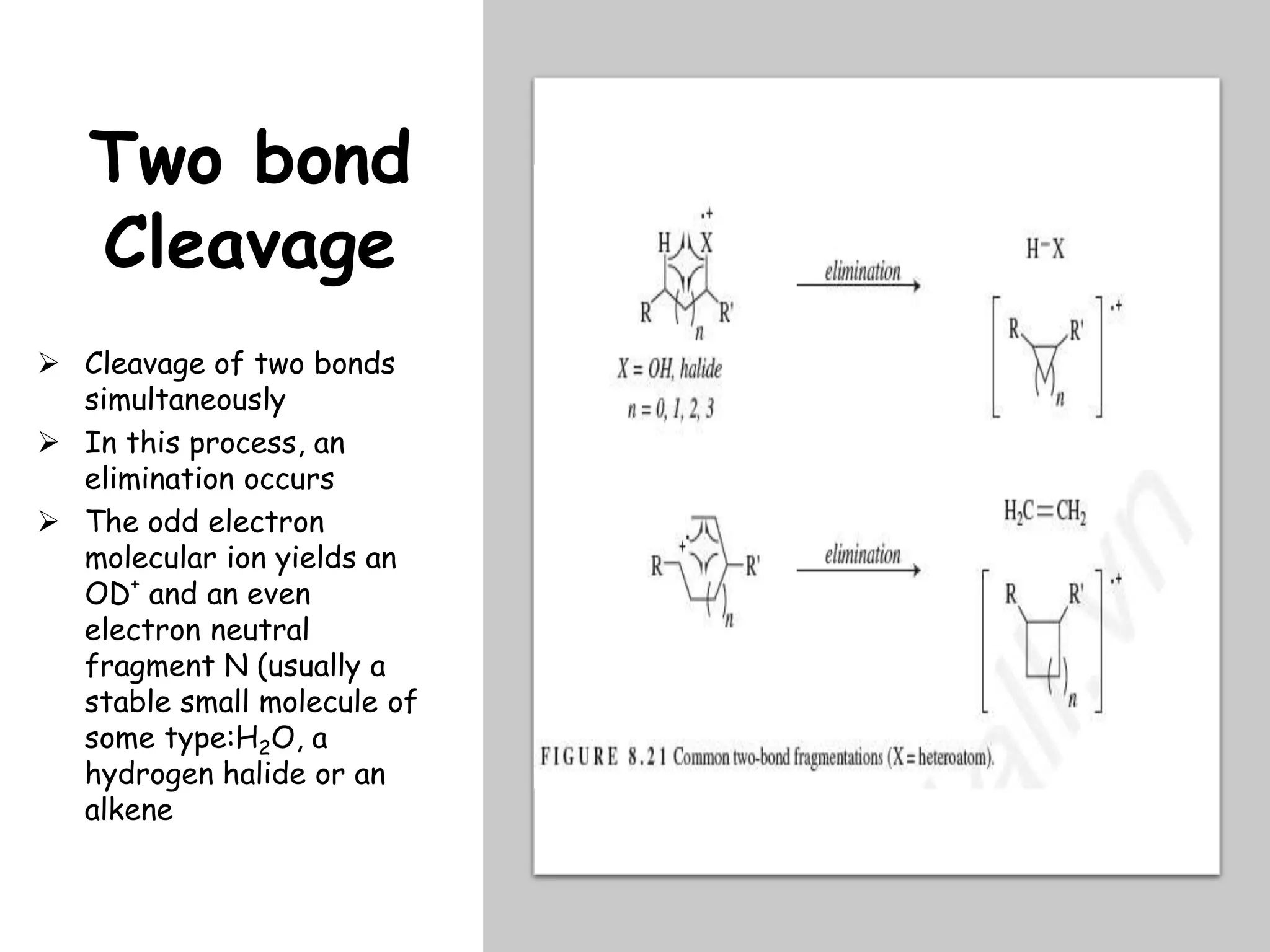
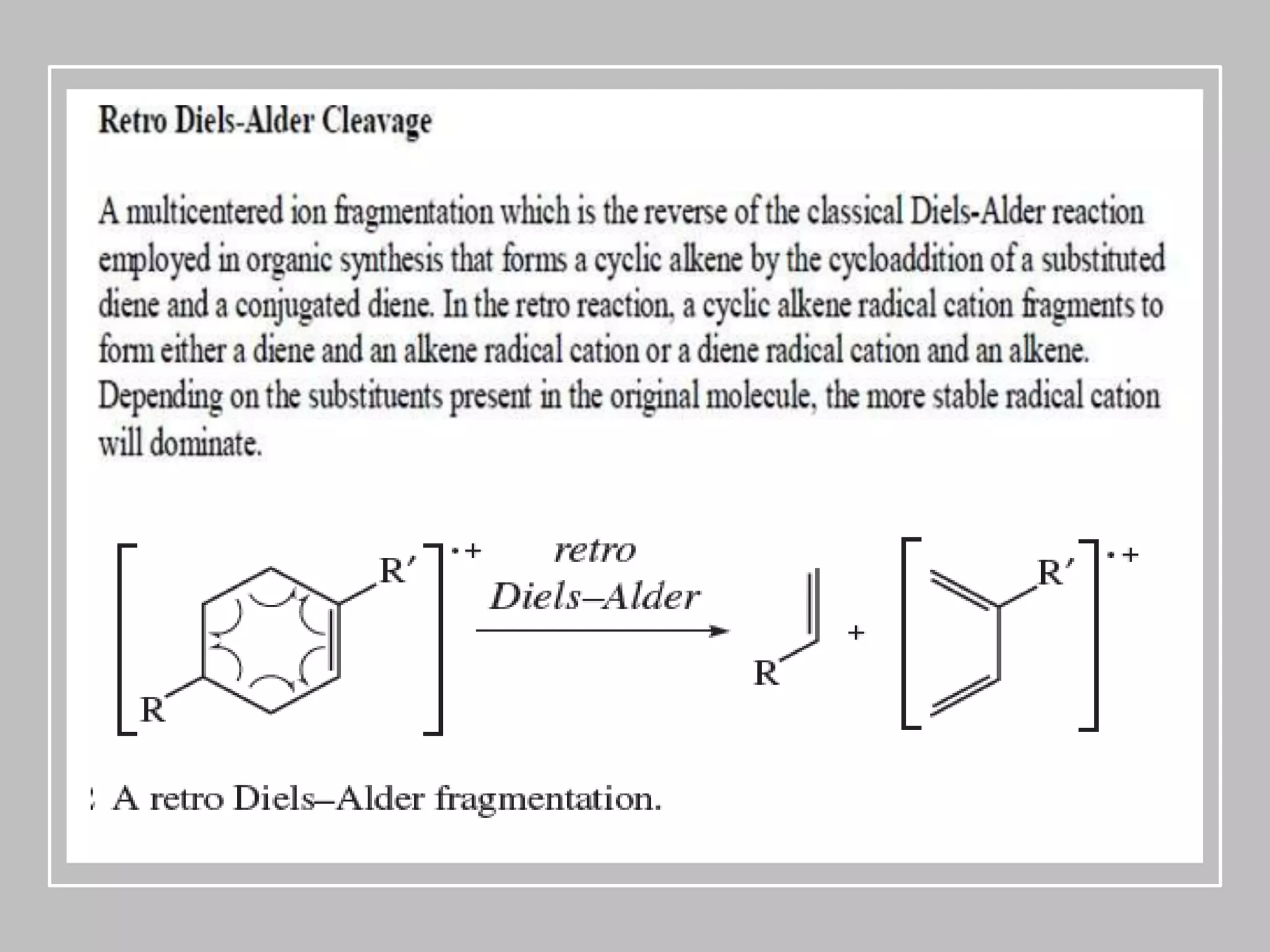
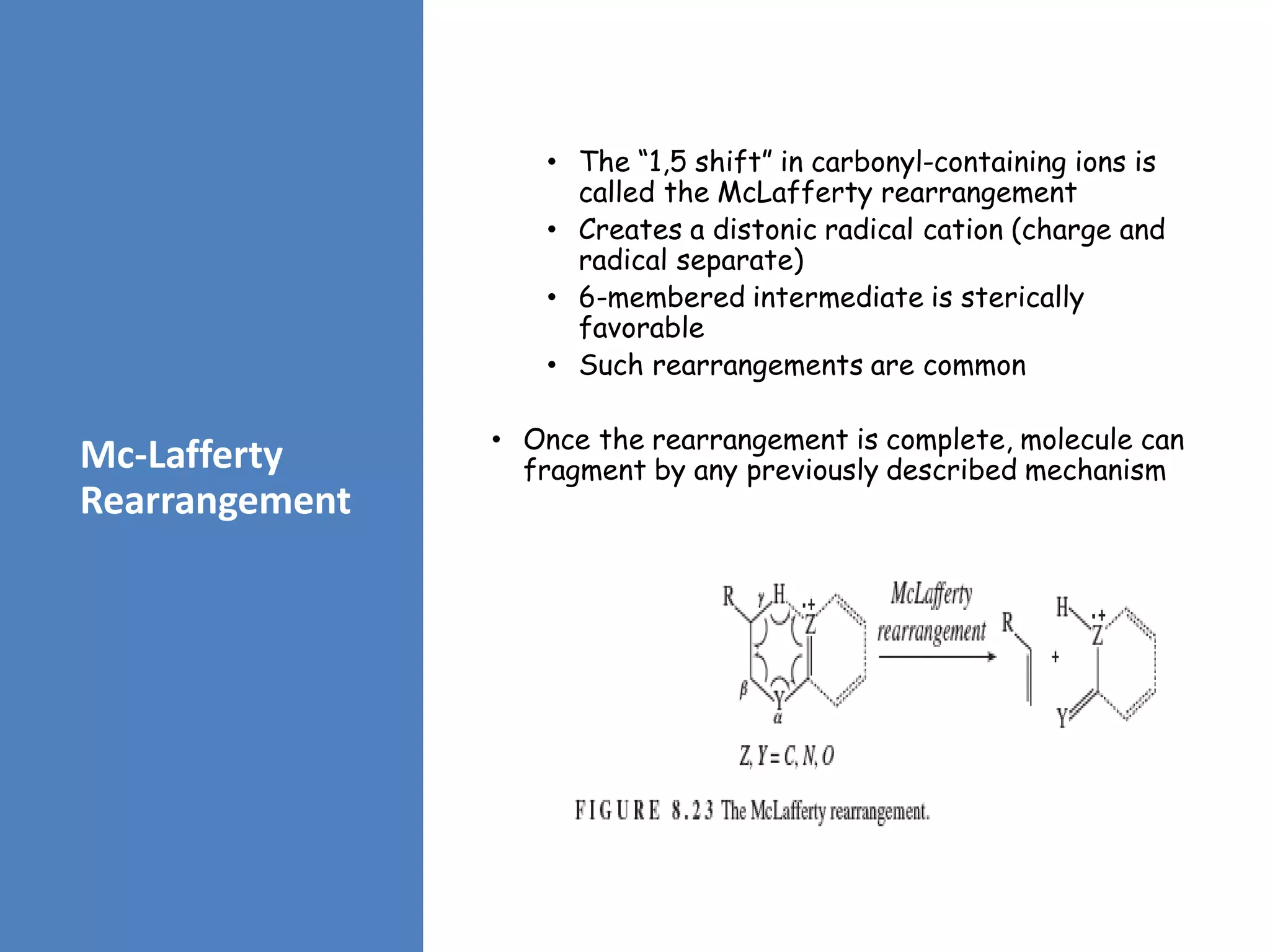
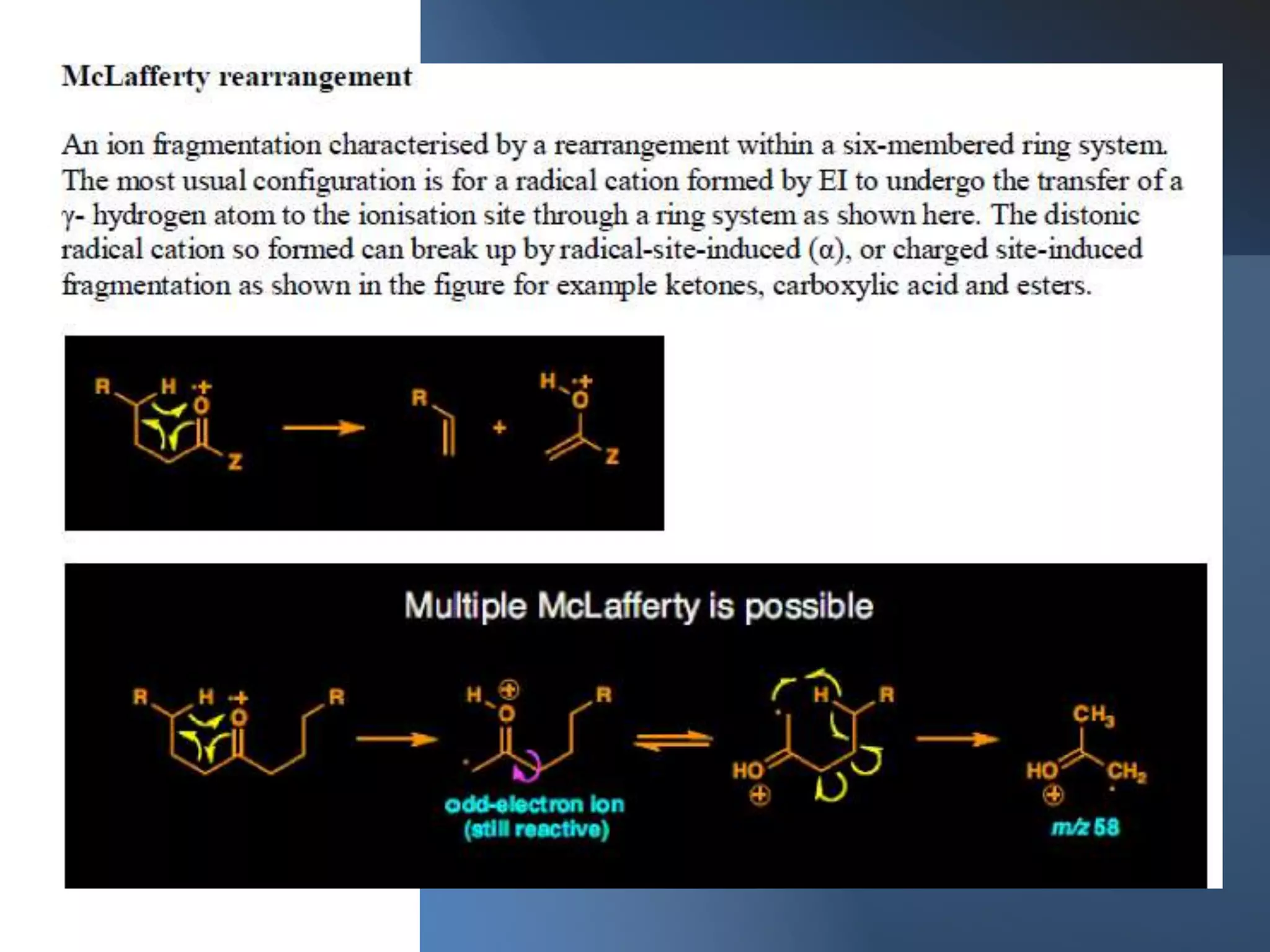
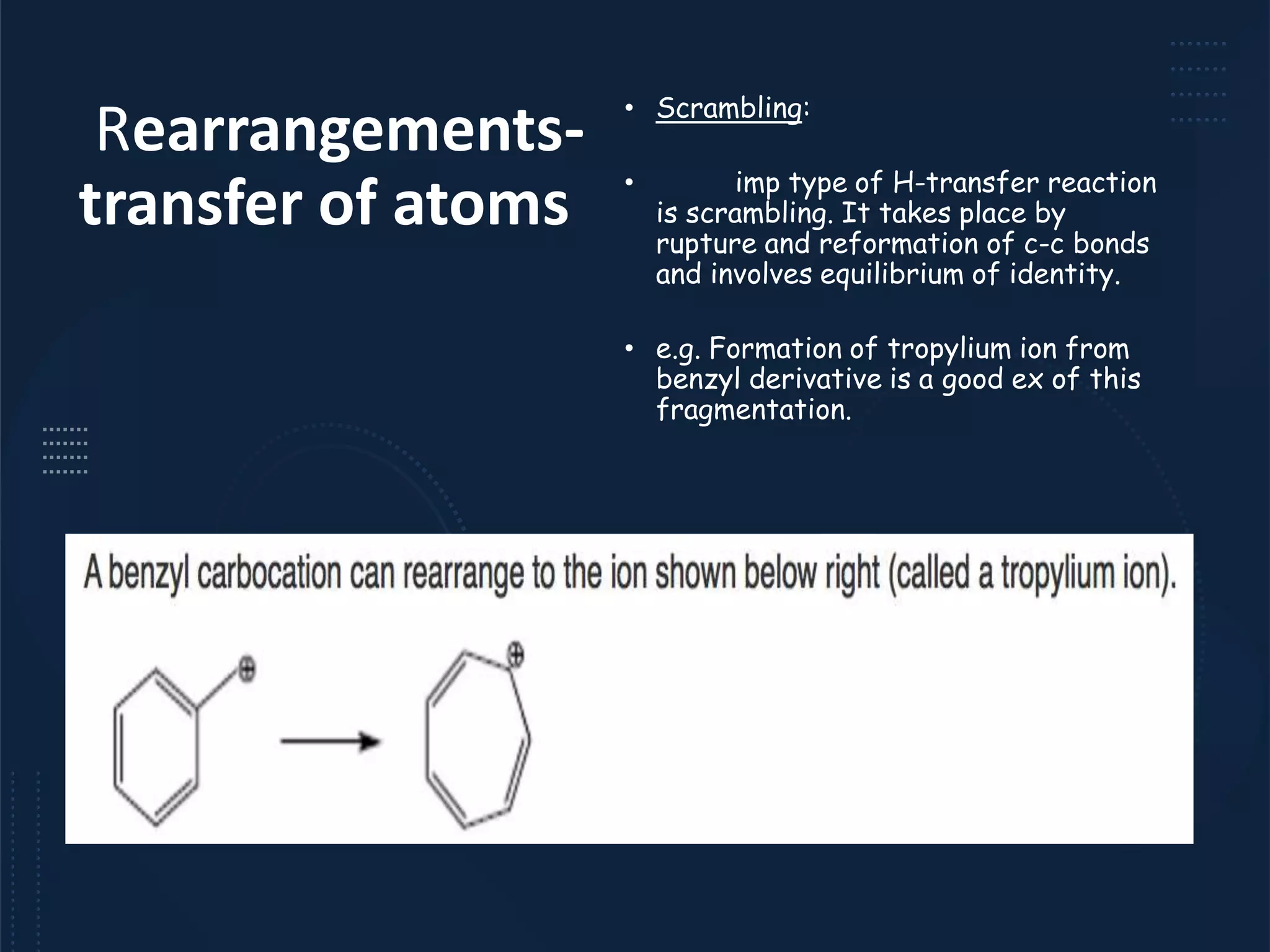
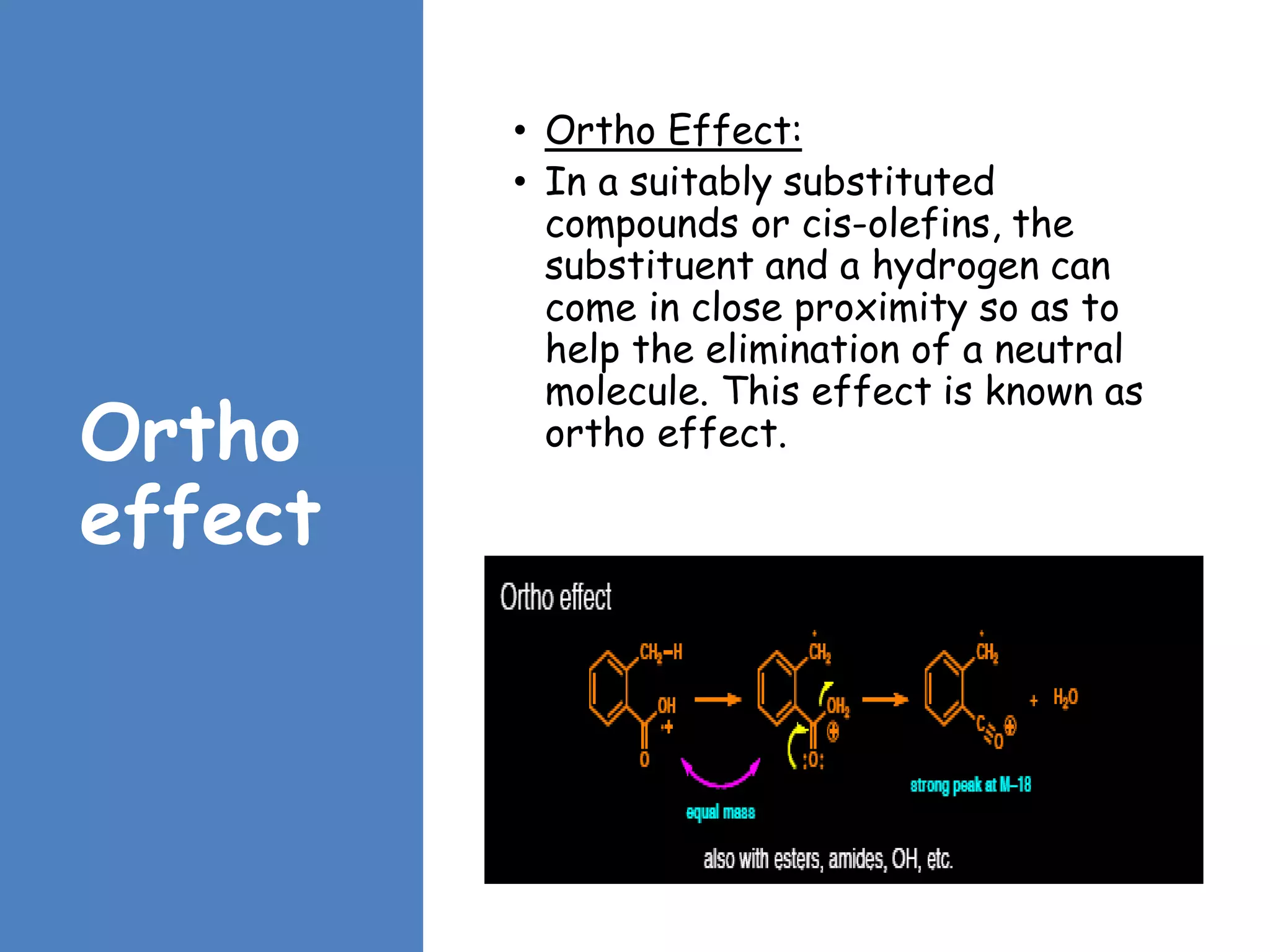
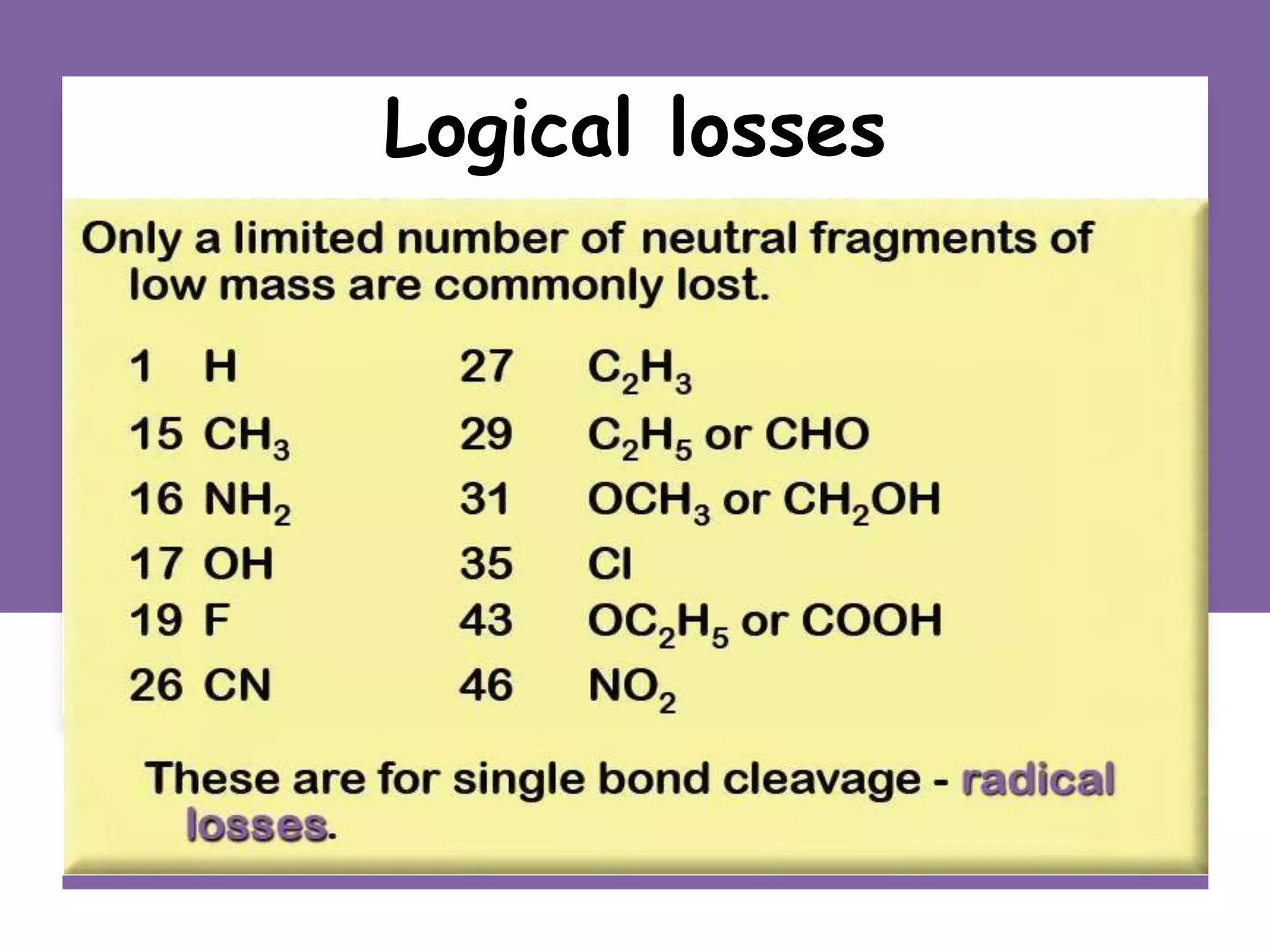
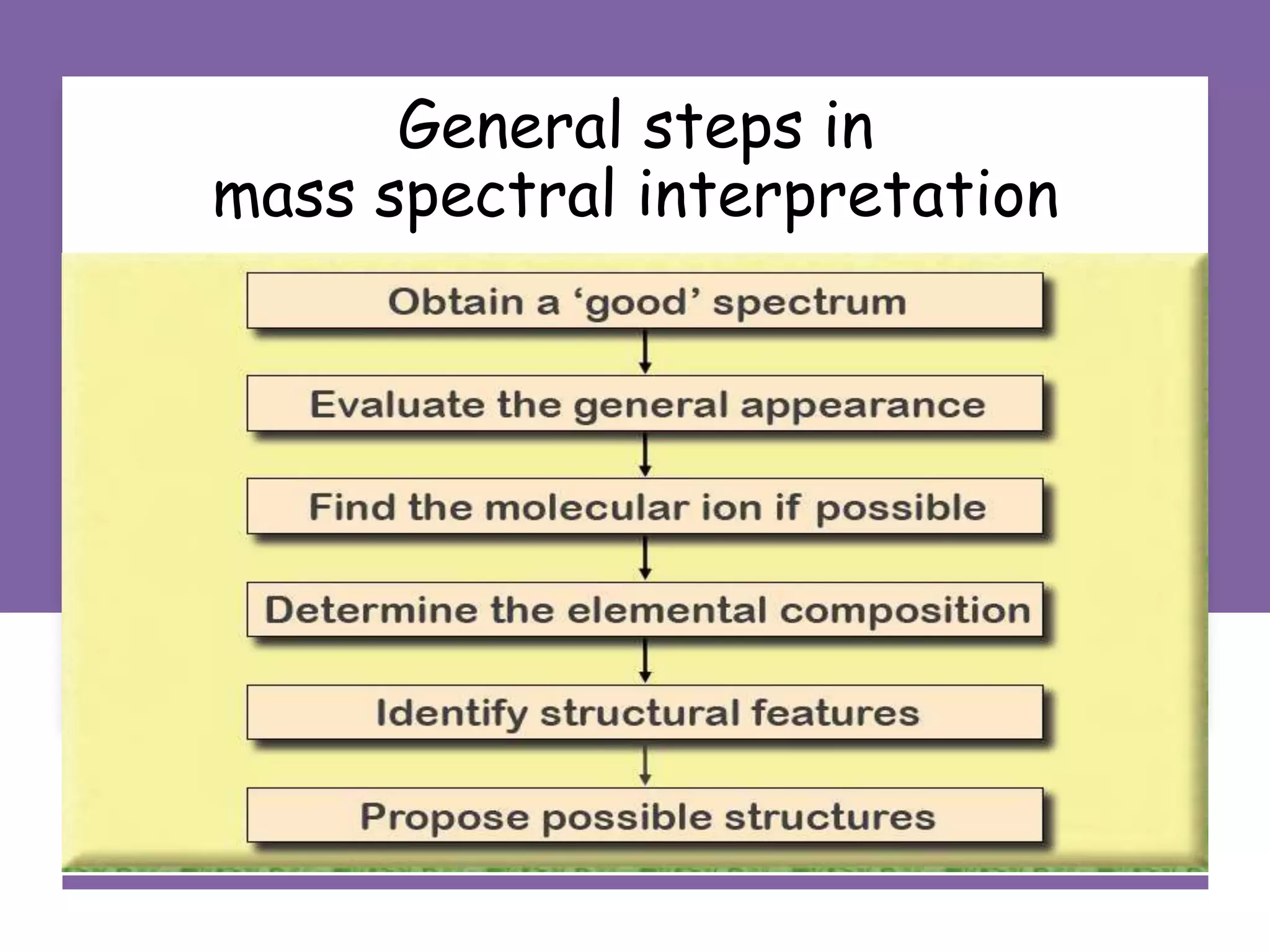
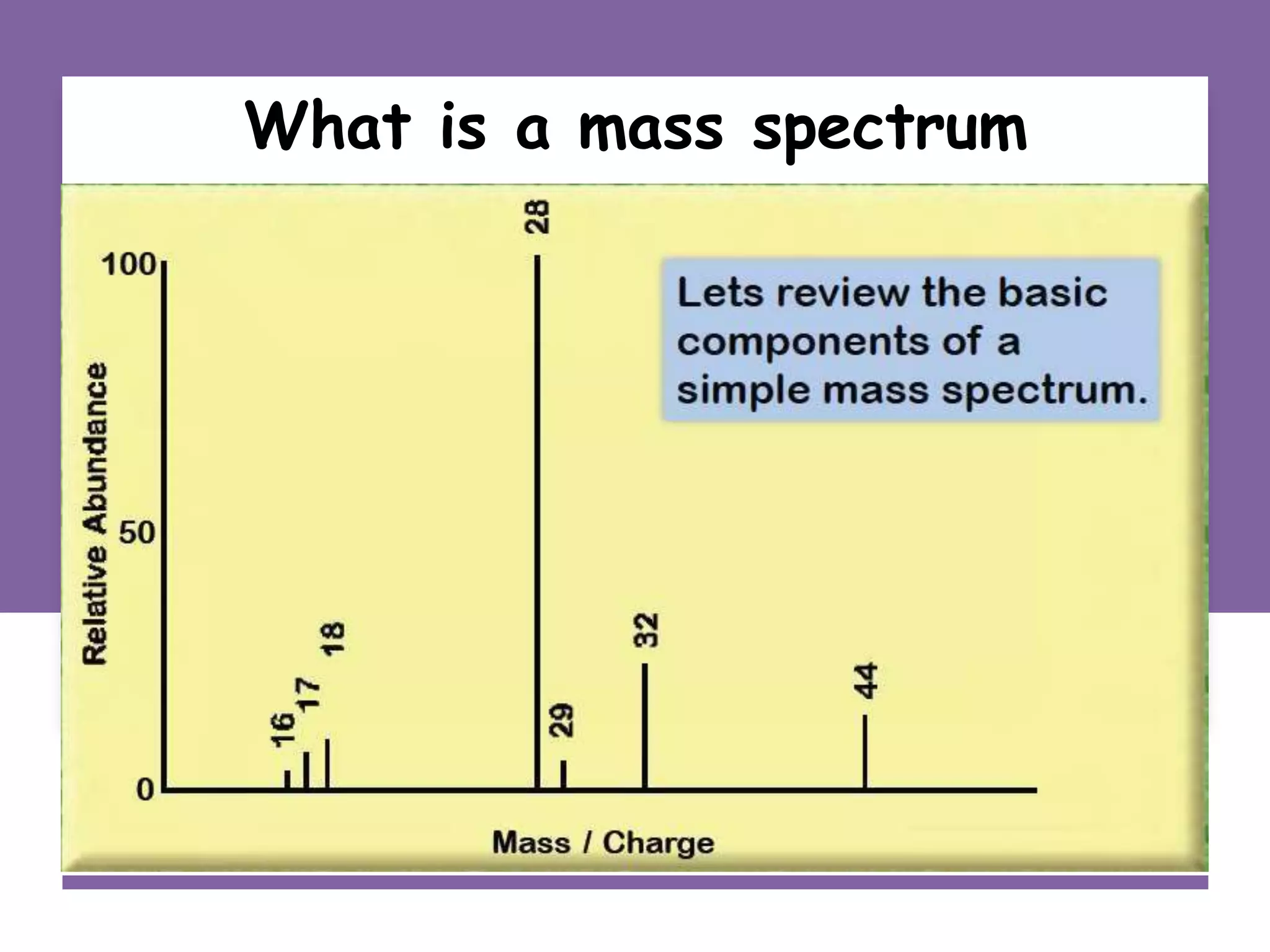
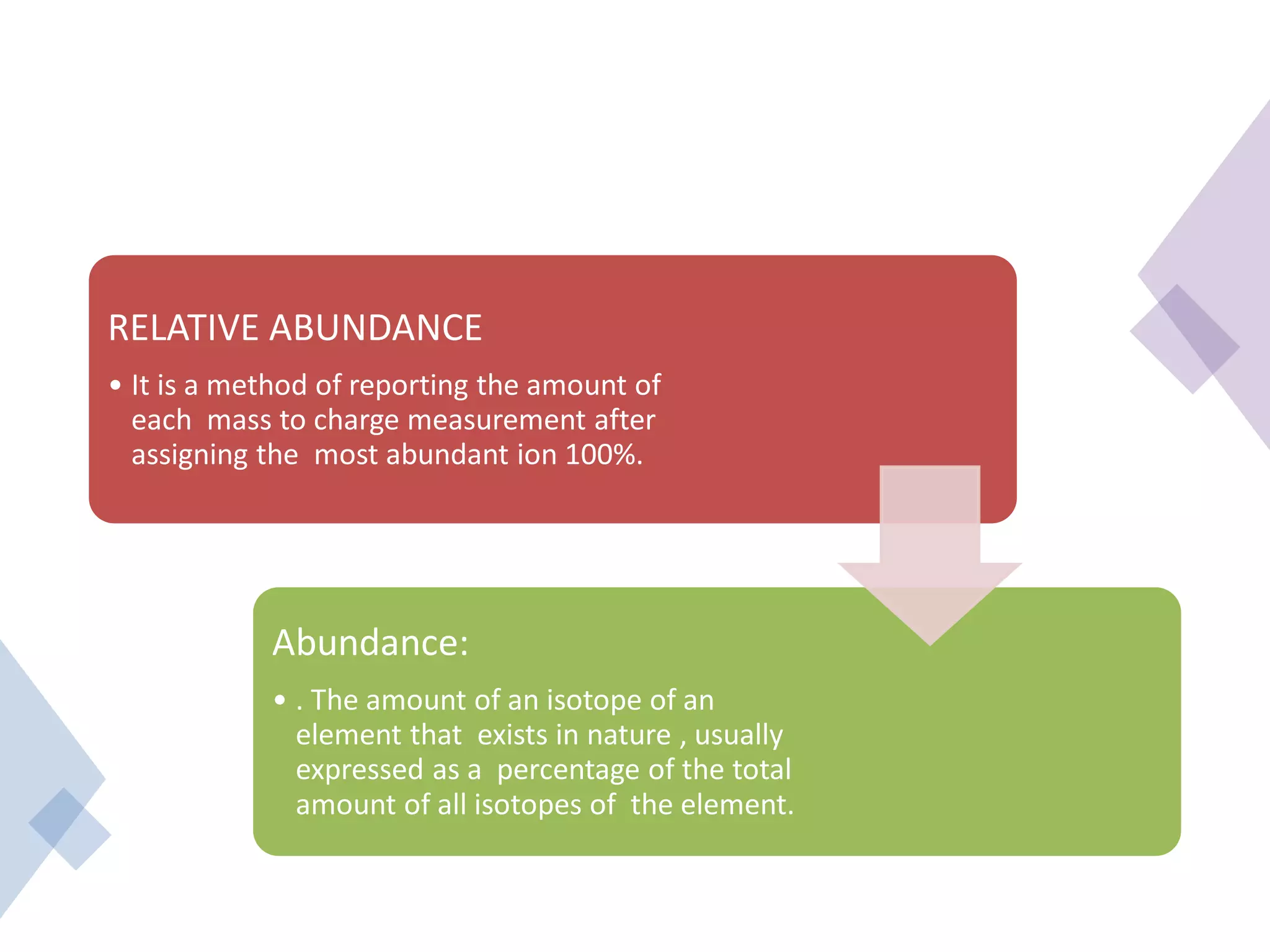
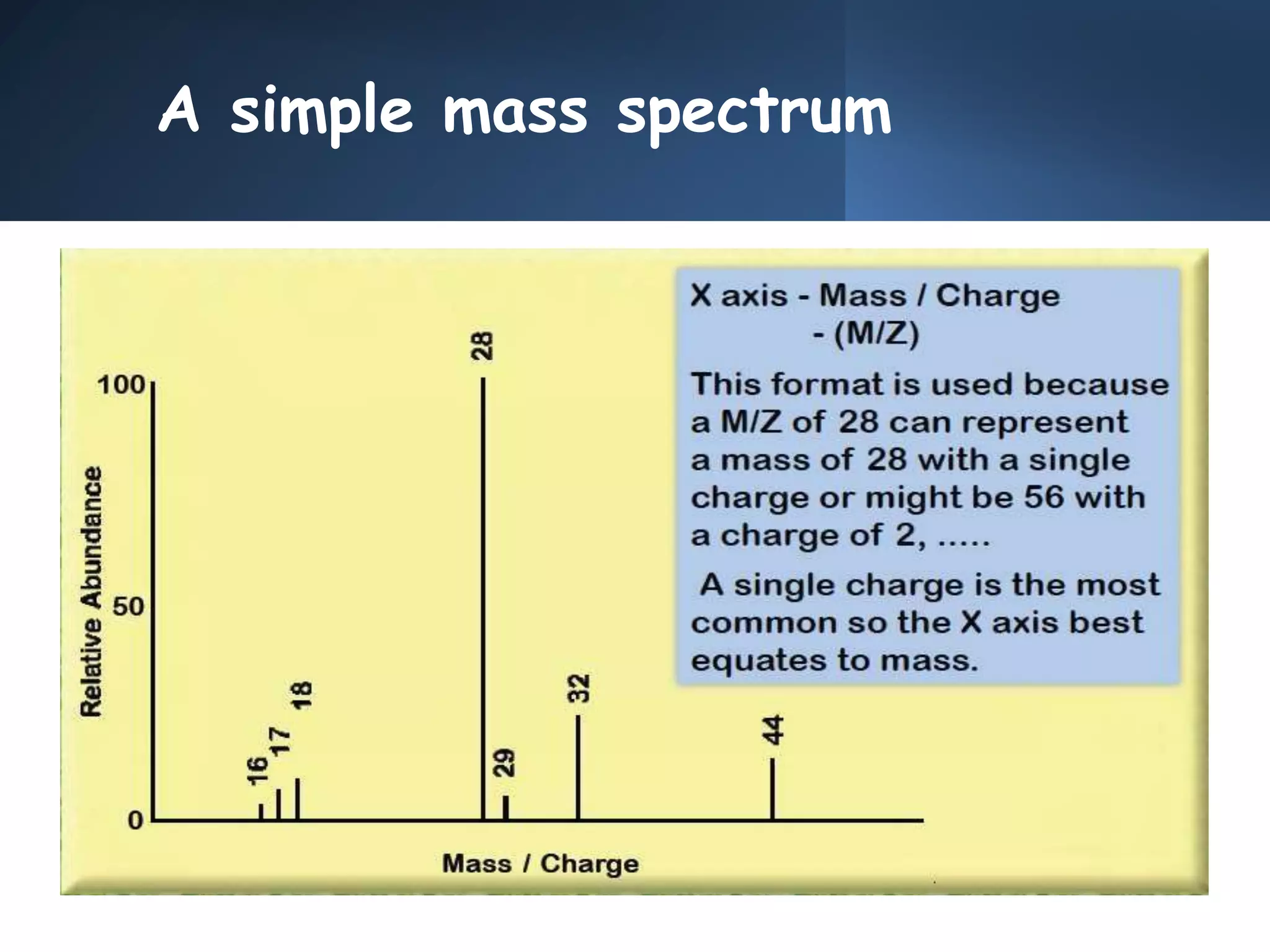
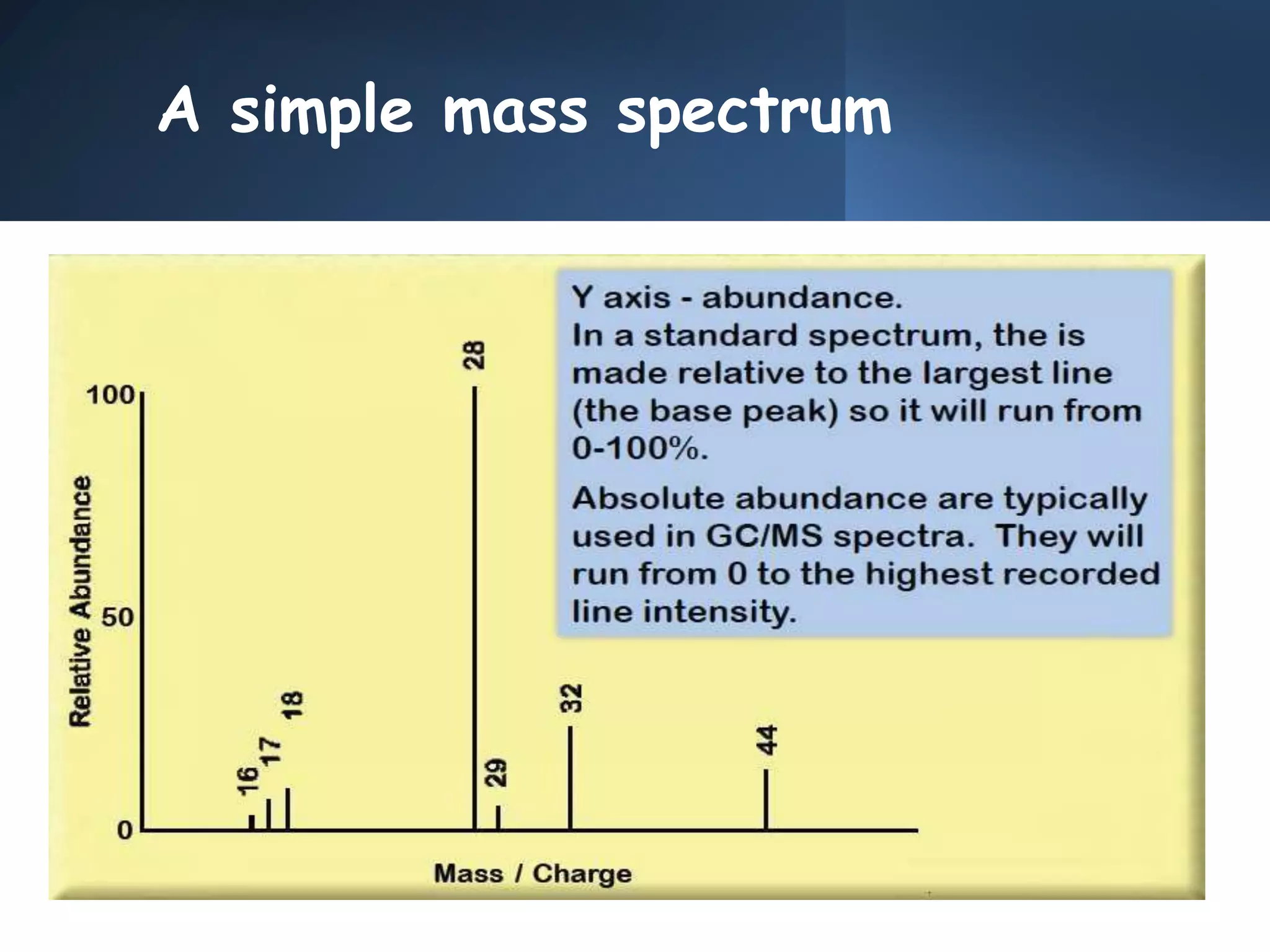
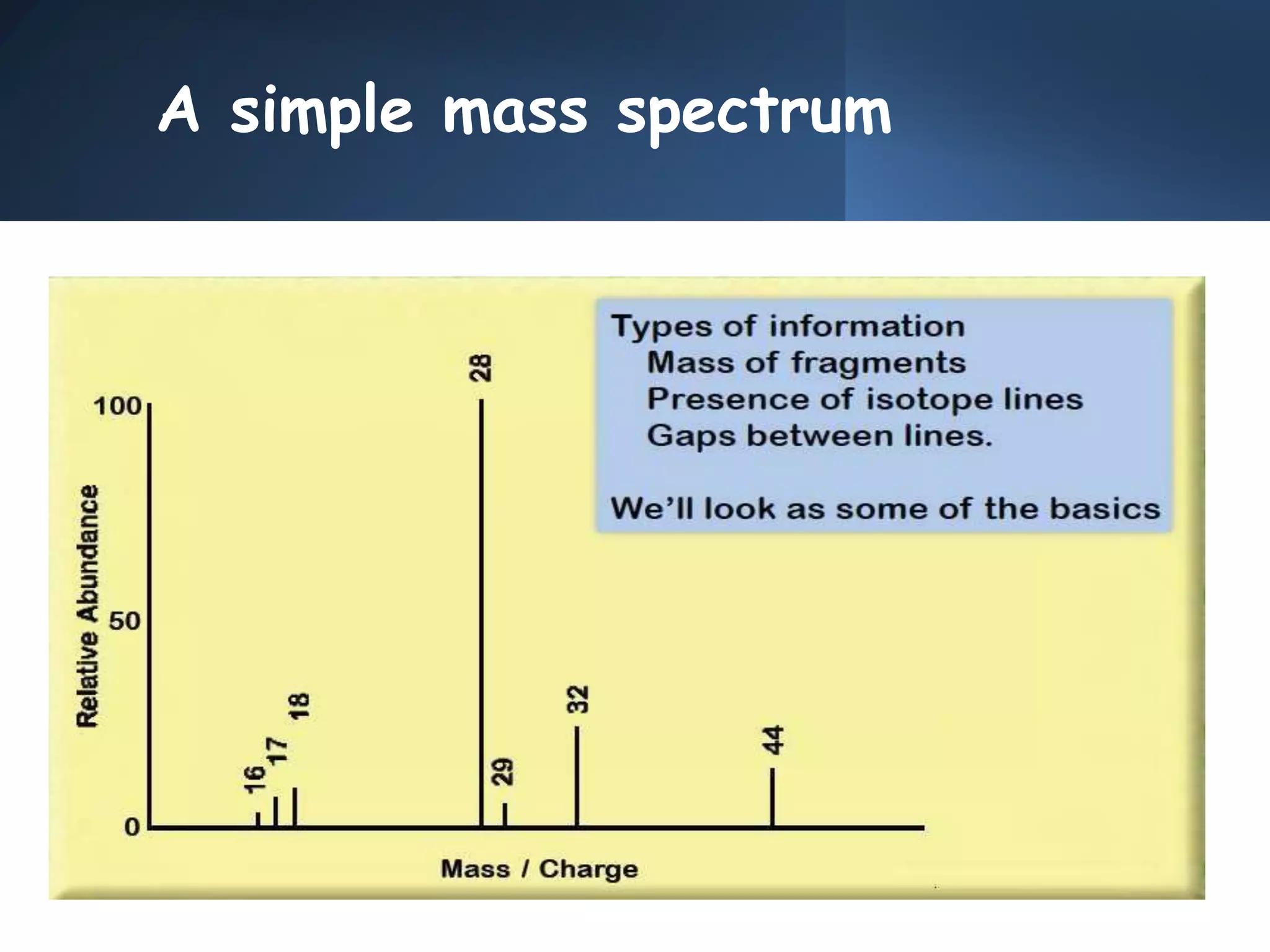
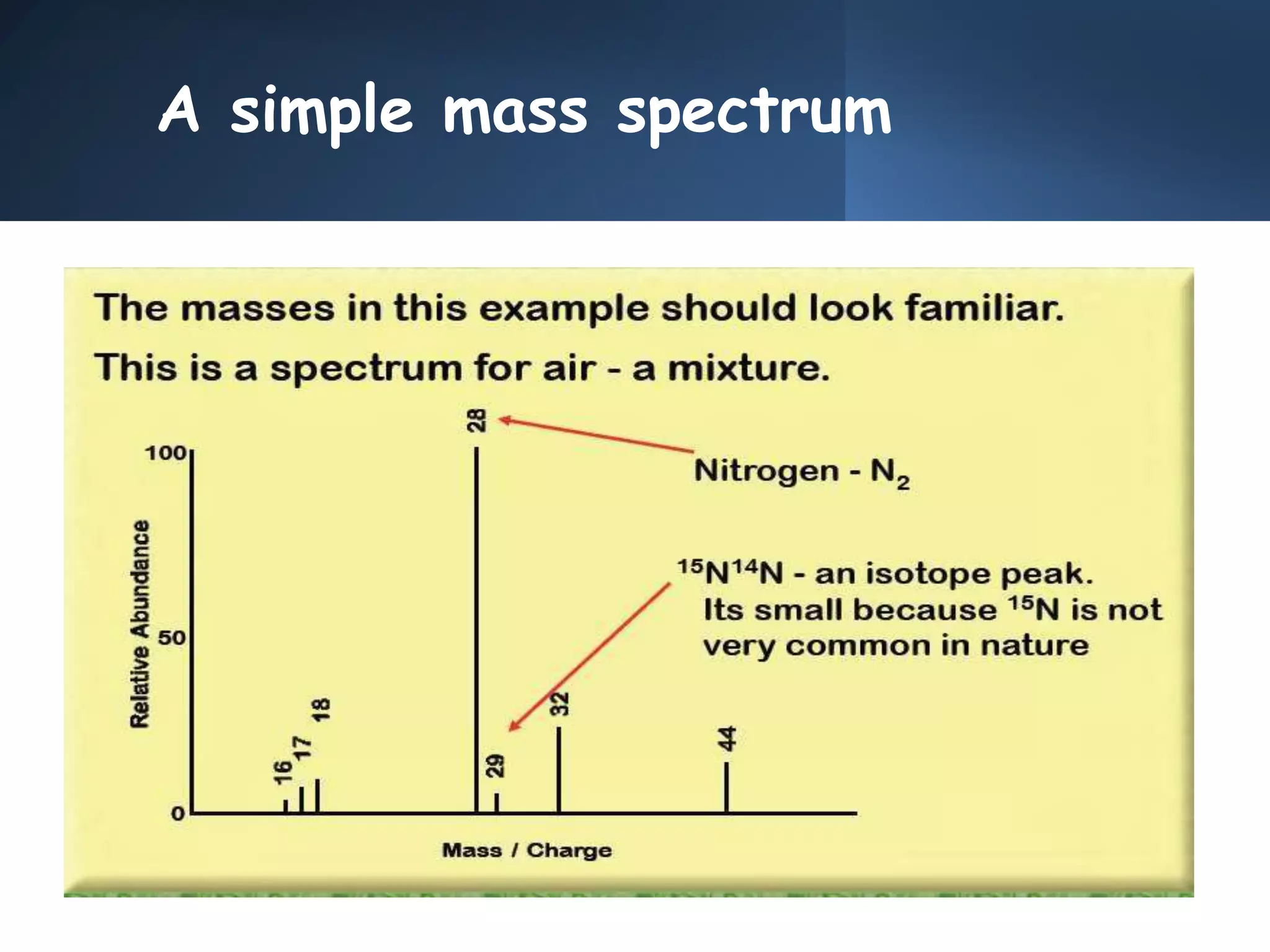
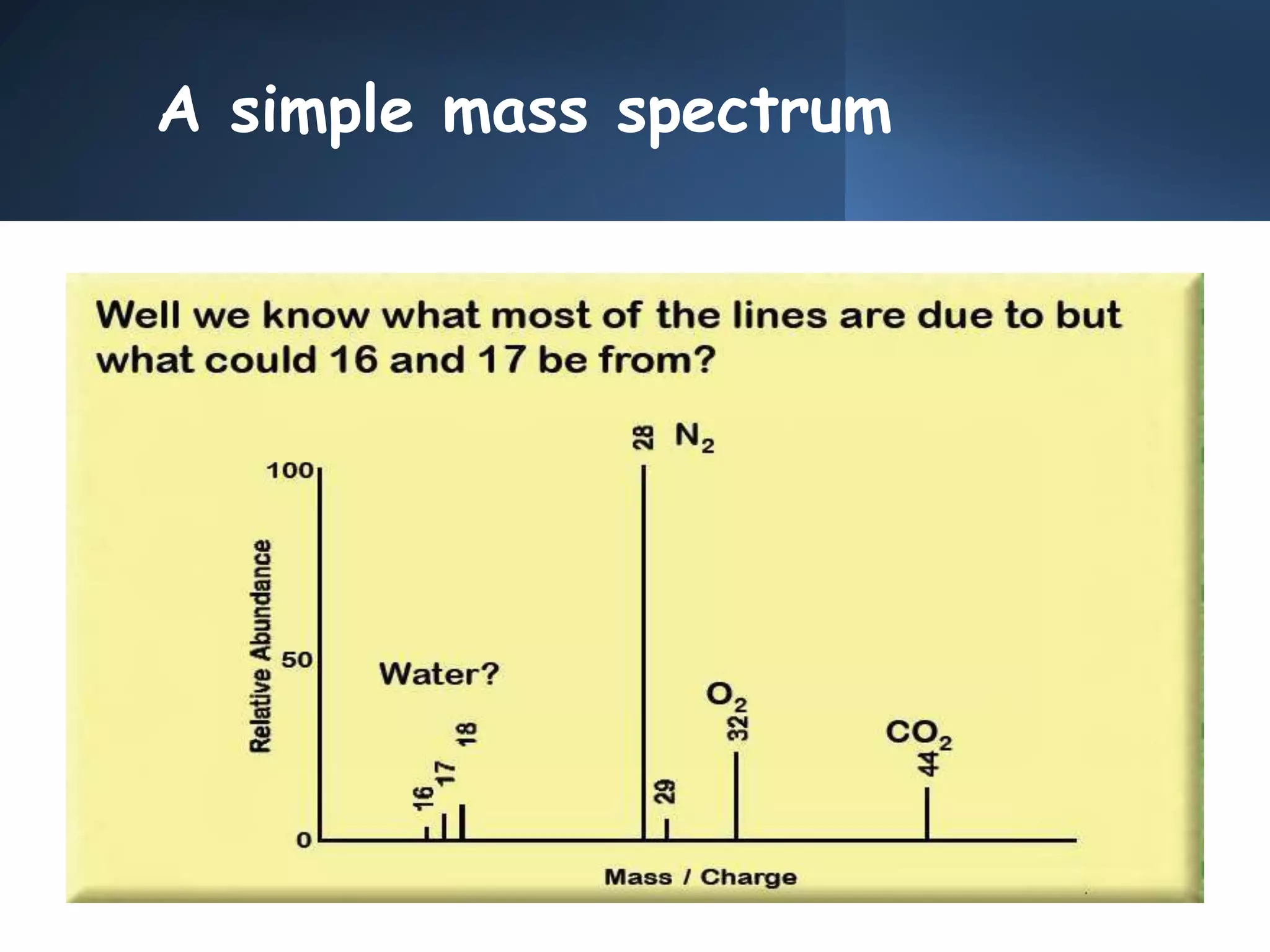
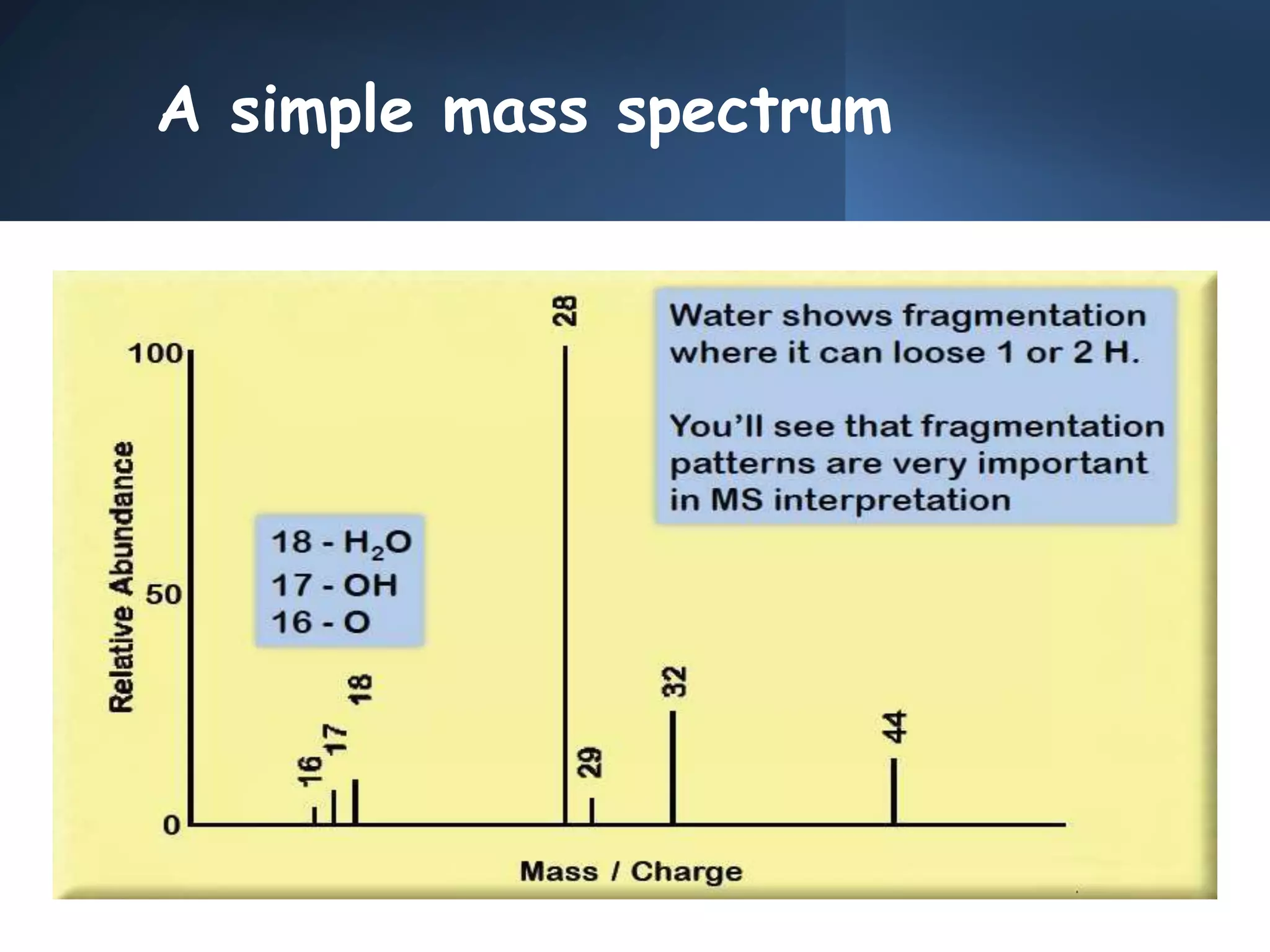
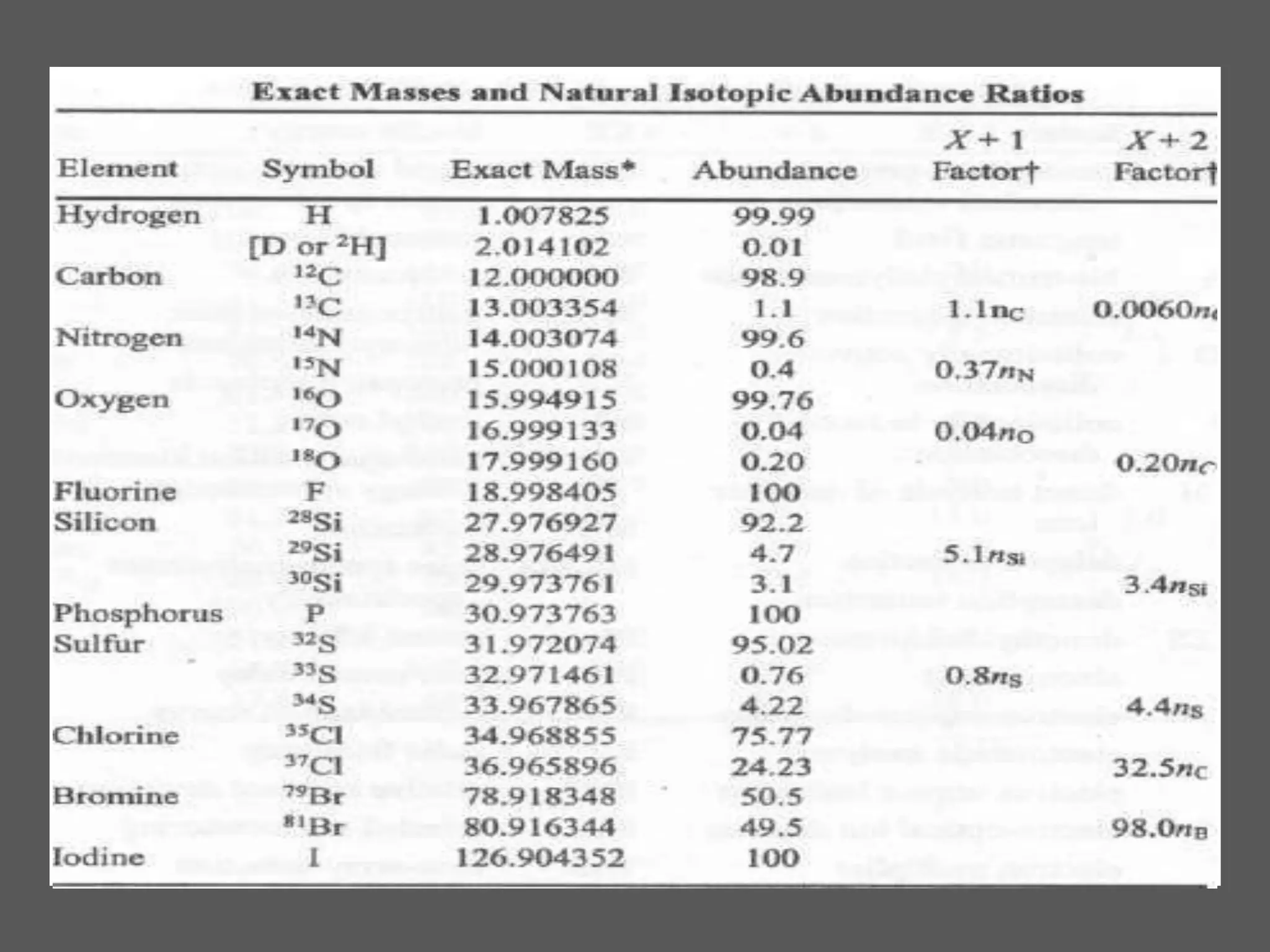
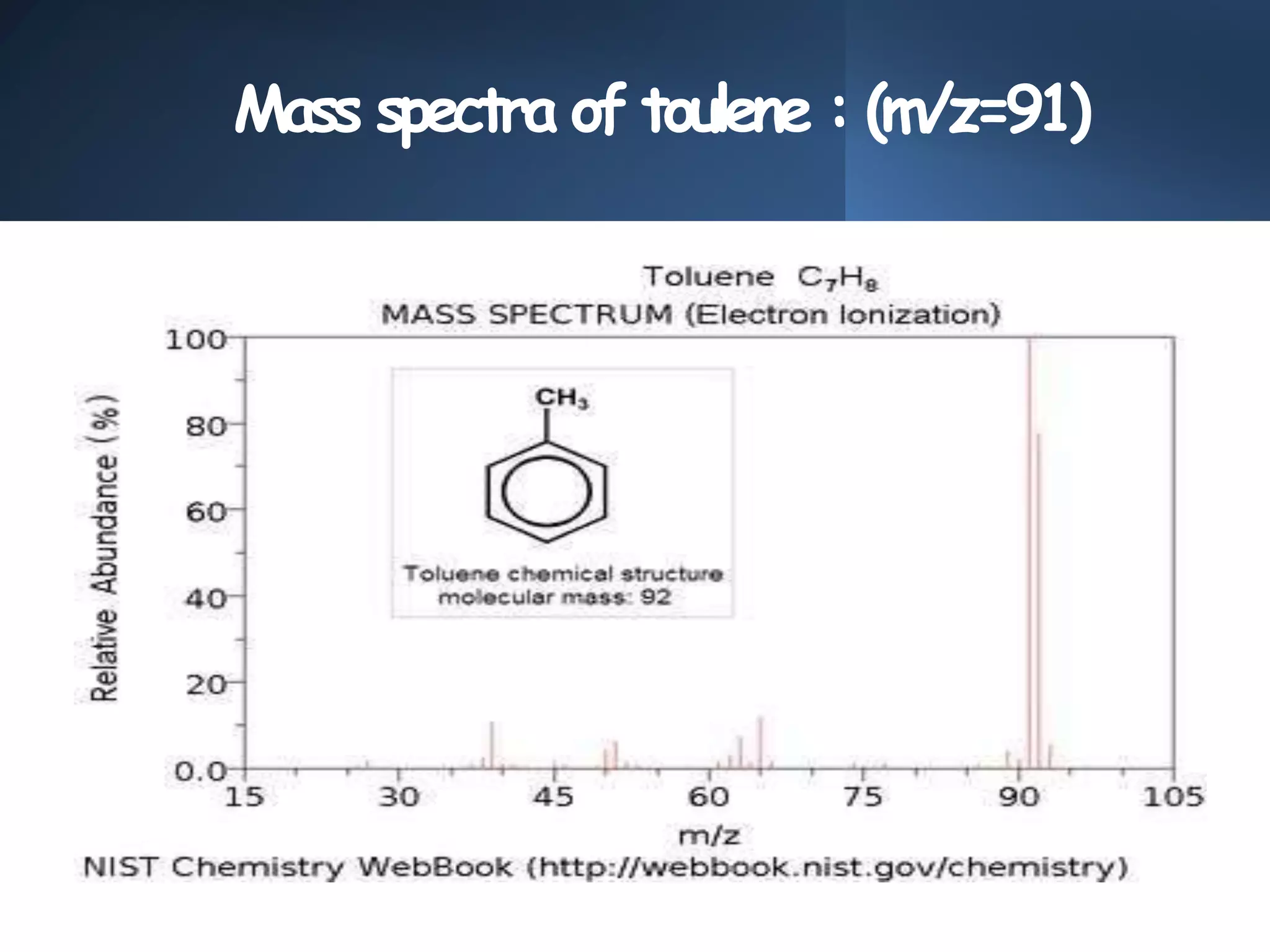
This document provides an overview of mass spectrometry, including its basic principles, fragmentation patterns, and interpretation of mass spectra. It discusses the formation of molecular ions and their fragments, different cleavage mechanisms, and key rearrangement reactions relevant to mass spectrometry. Additionally, it addresses the isotopic effects and the nitrogen rule related to molecular weight determination.