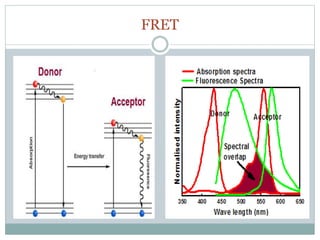
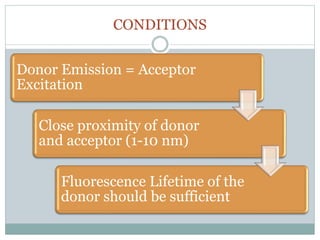
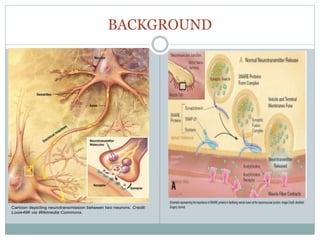
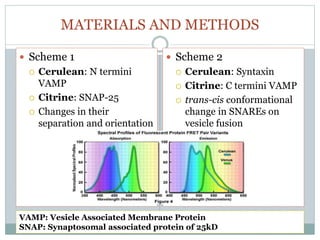
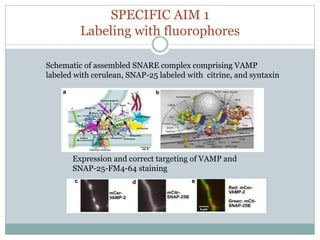
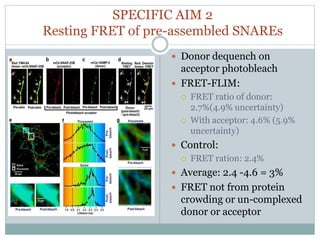
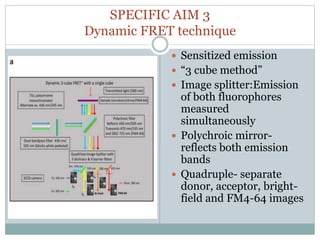
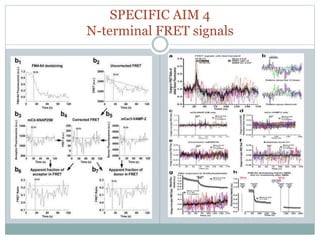
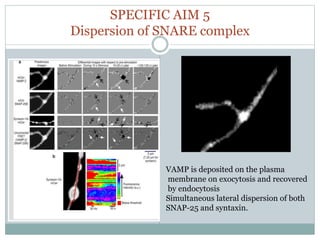
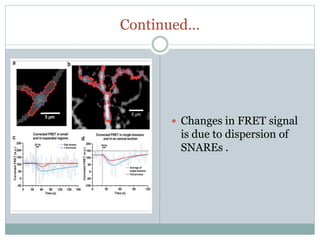
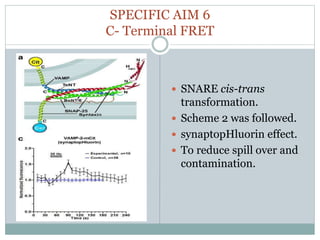
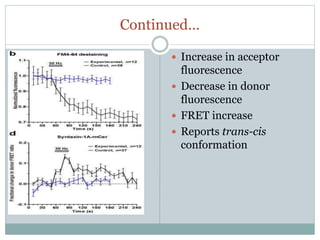
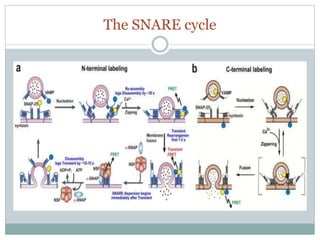
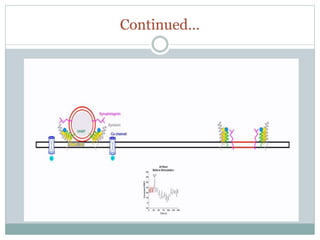
This document discusses using fluorescence resonance energy transfer (FRET) to study the assembly and rearrangements of SNARE proteins during exocytosis at neural synapses. It describes two schemes using FRET to label specific SNARE proteins with donor and acceptor fluorophores. Scheme 1 uses Cerulean and Citrine labels on VAMP and SNAP-25 to detect resting SNARE complexes and their dispersion after vesicle fusion. Scheme 2 uses Cerulean on Syntaxin and Citrine on VAMP to monitor the trans-cis conformational change in SNAREs. The study finds FRET signals indicating the dynamic rearrangement of pre-assembled SNARE complexes during different stages of the SNARE cycle.