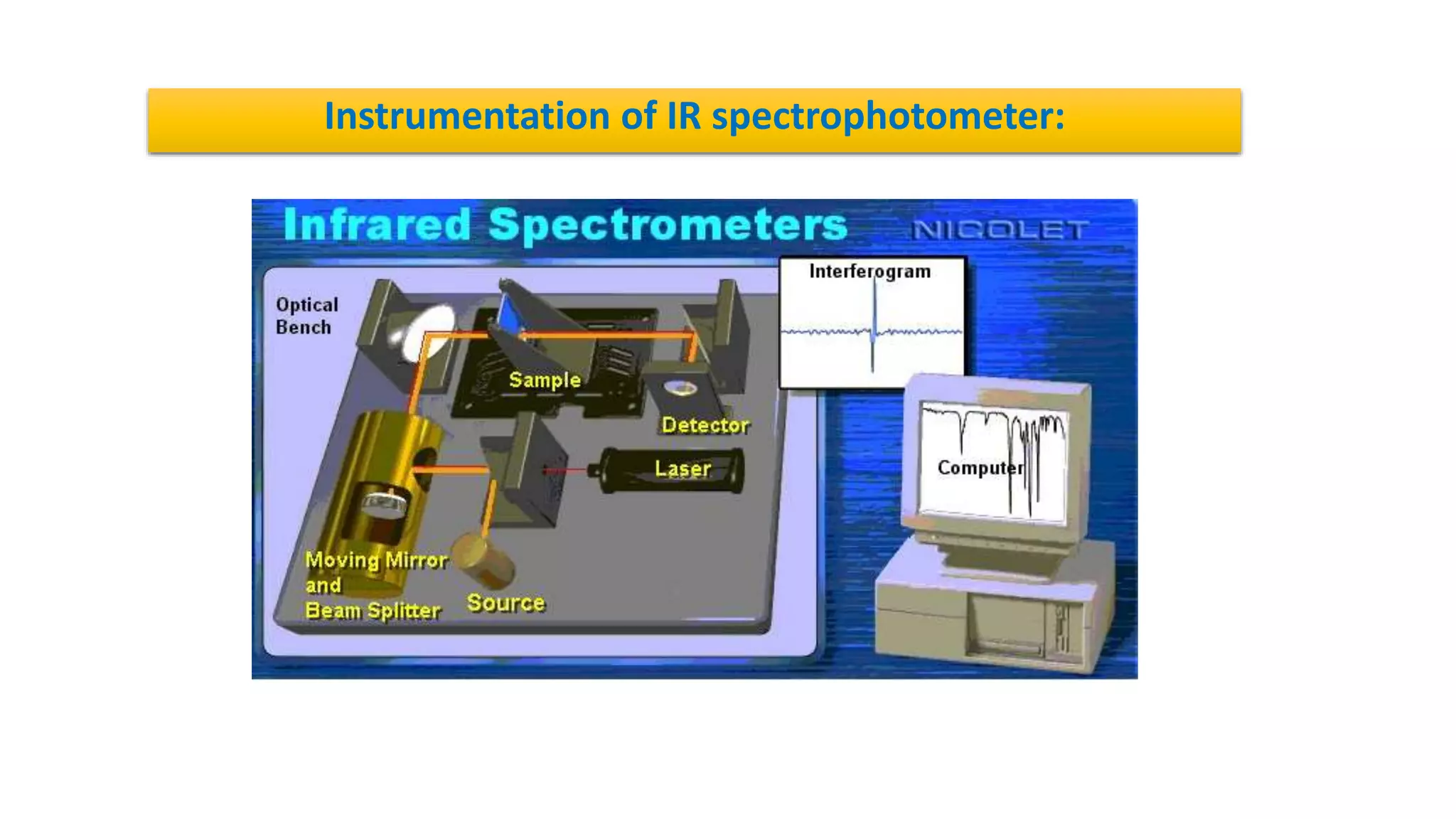
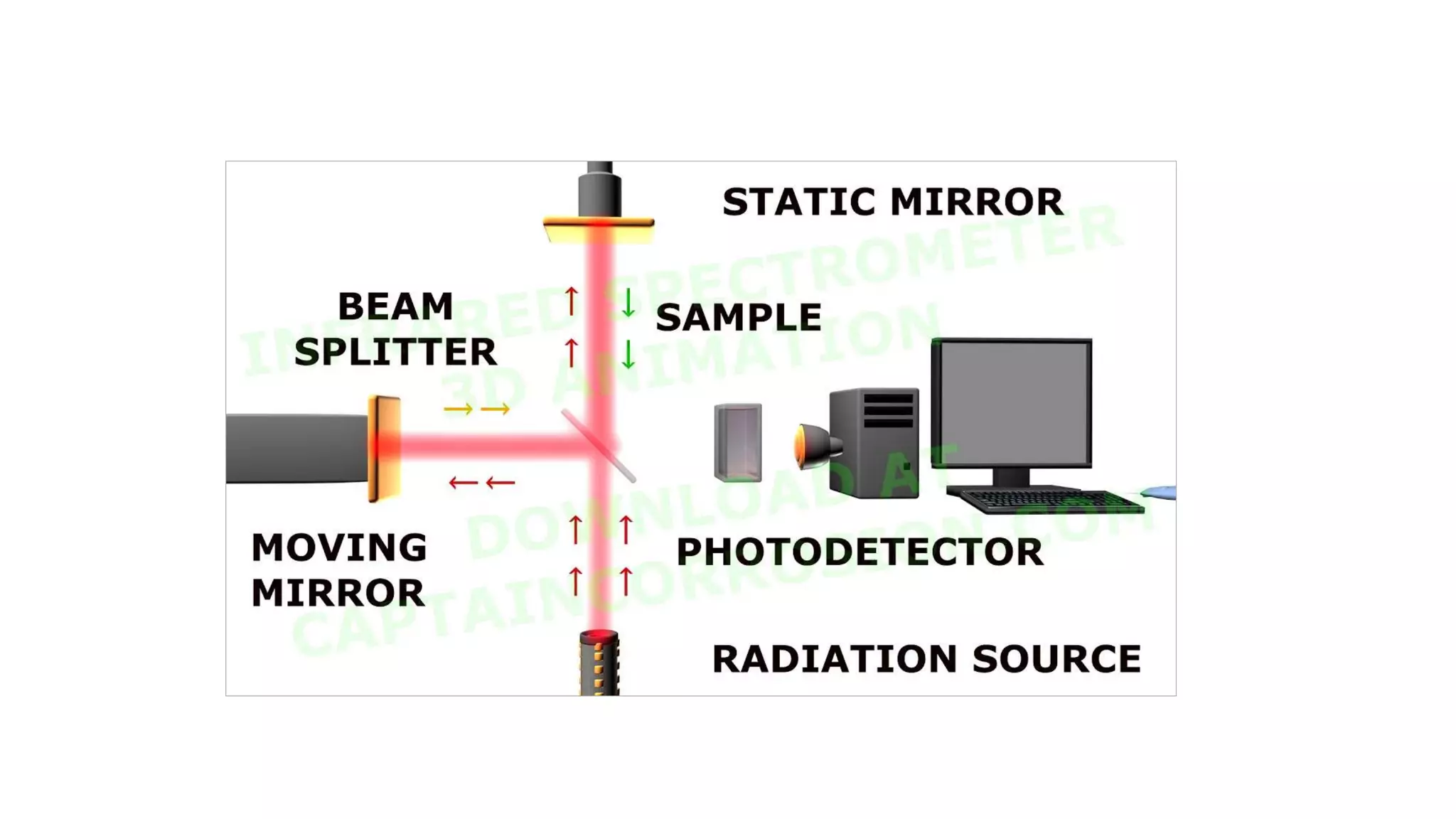
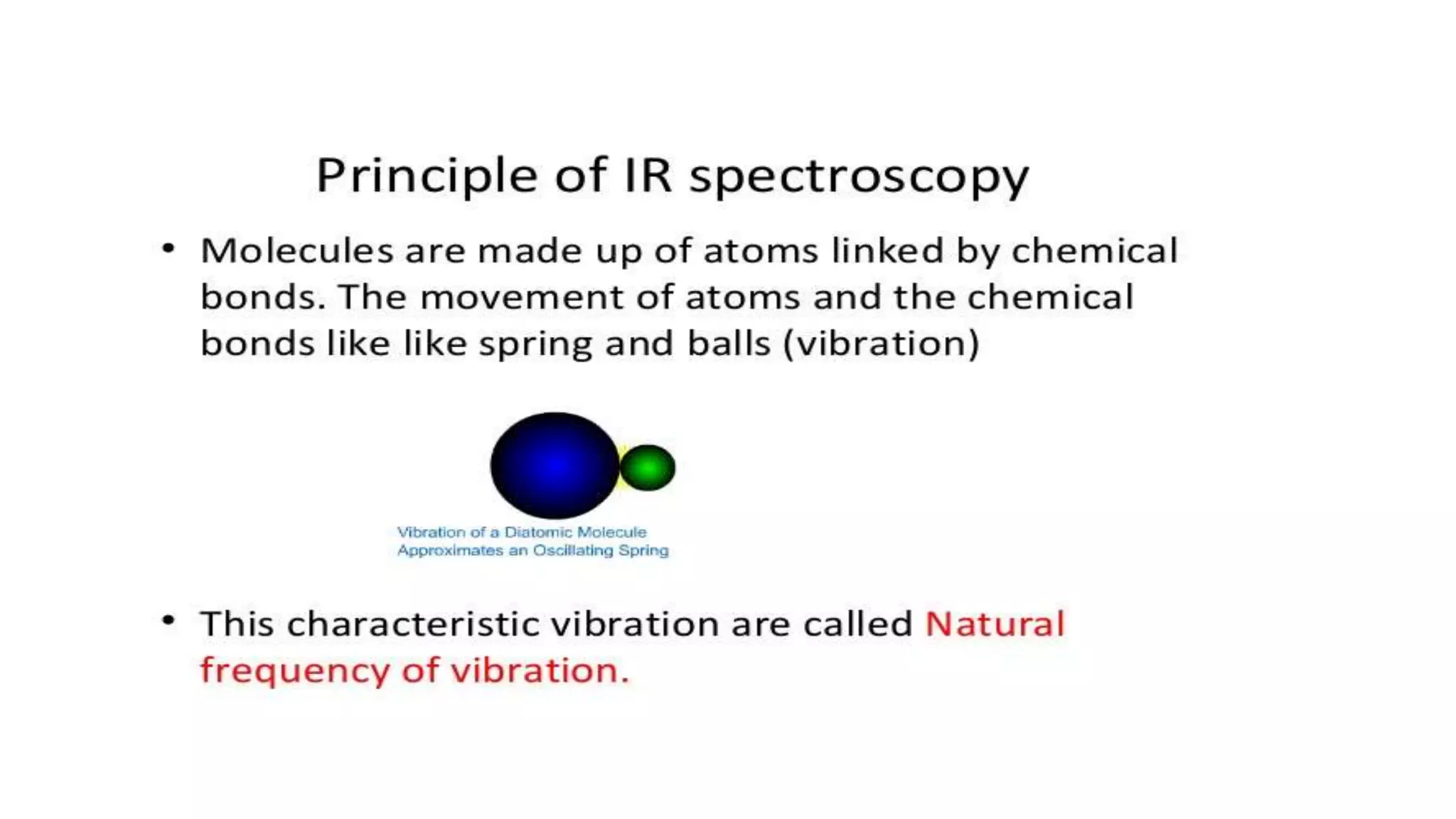
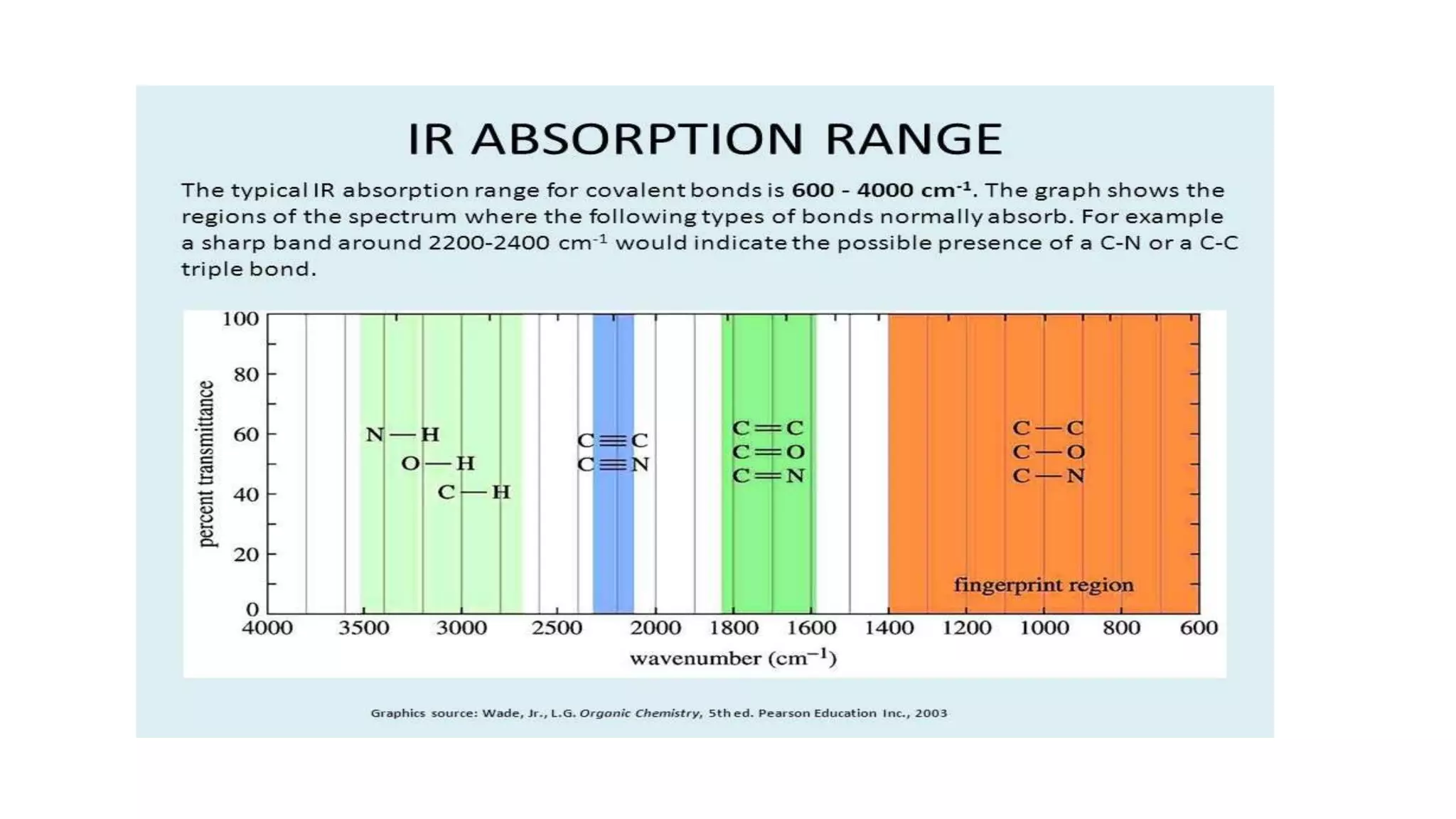
Infrared (IR) spectroscopy is a technique that uses infrared light to study molecular interactions, providing information about functional groups in molecules. The IR spectrum acts as a fingerprint for molecular identification, reliant on proper wavelength absorption and changes in dipole moments. The document explains molecular vibrations, including stretching and bending vibrations, and emphasizes the significance of the middle IR region for analytical applications.