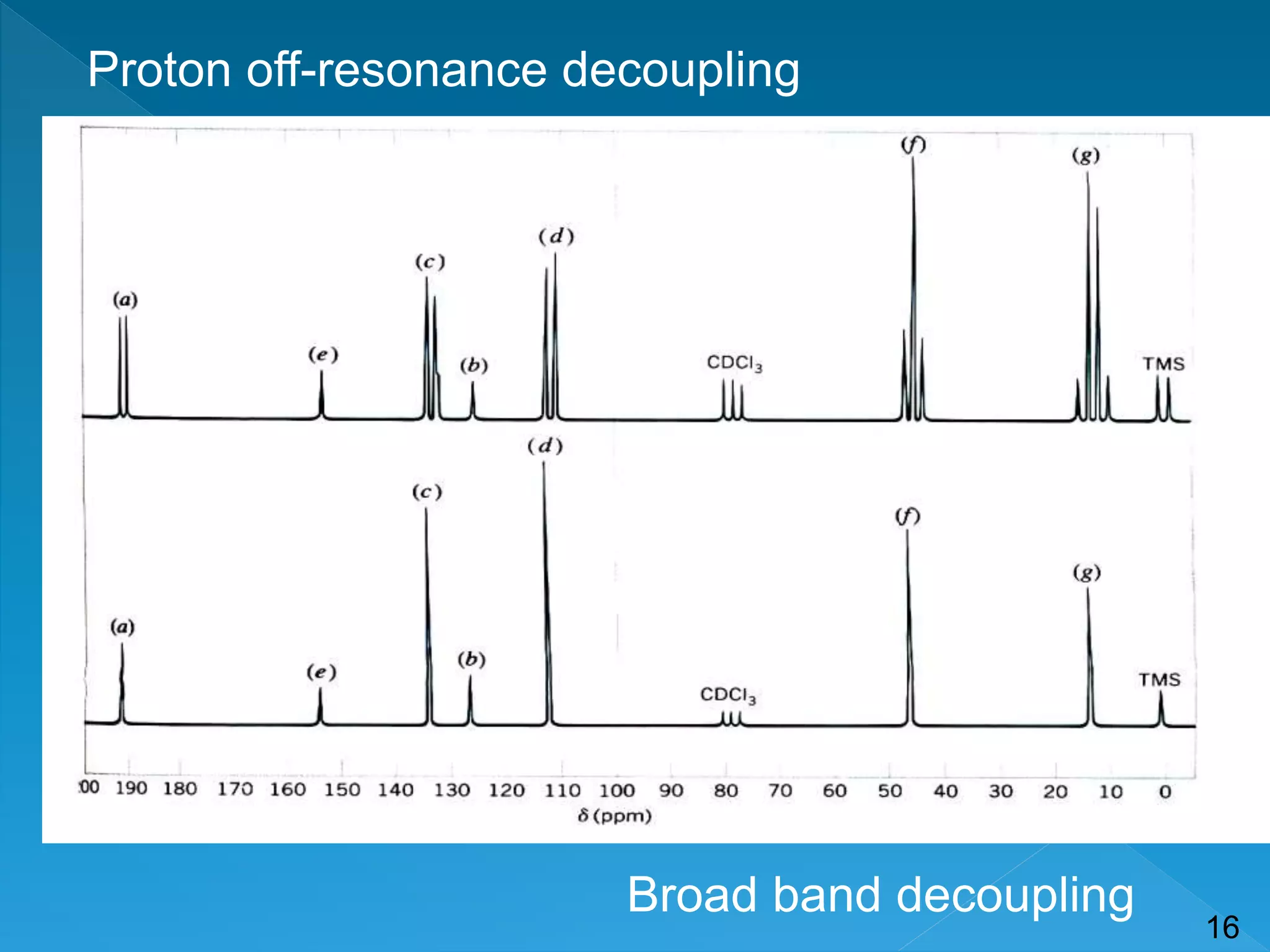
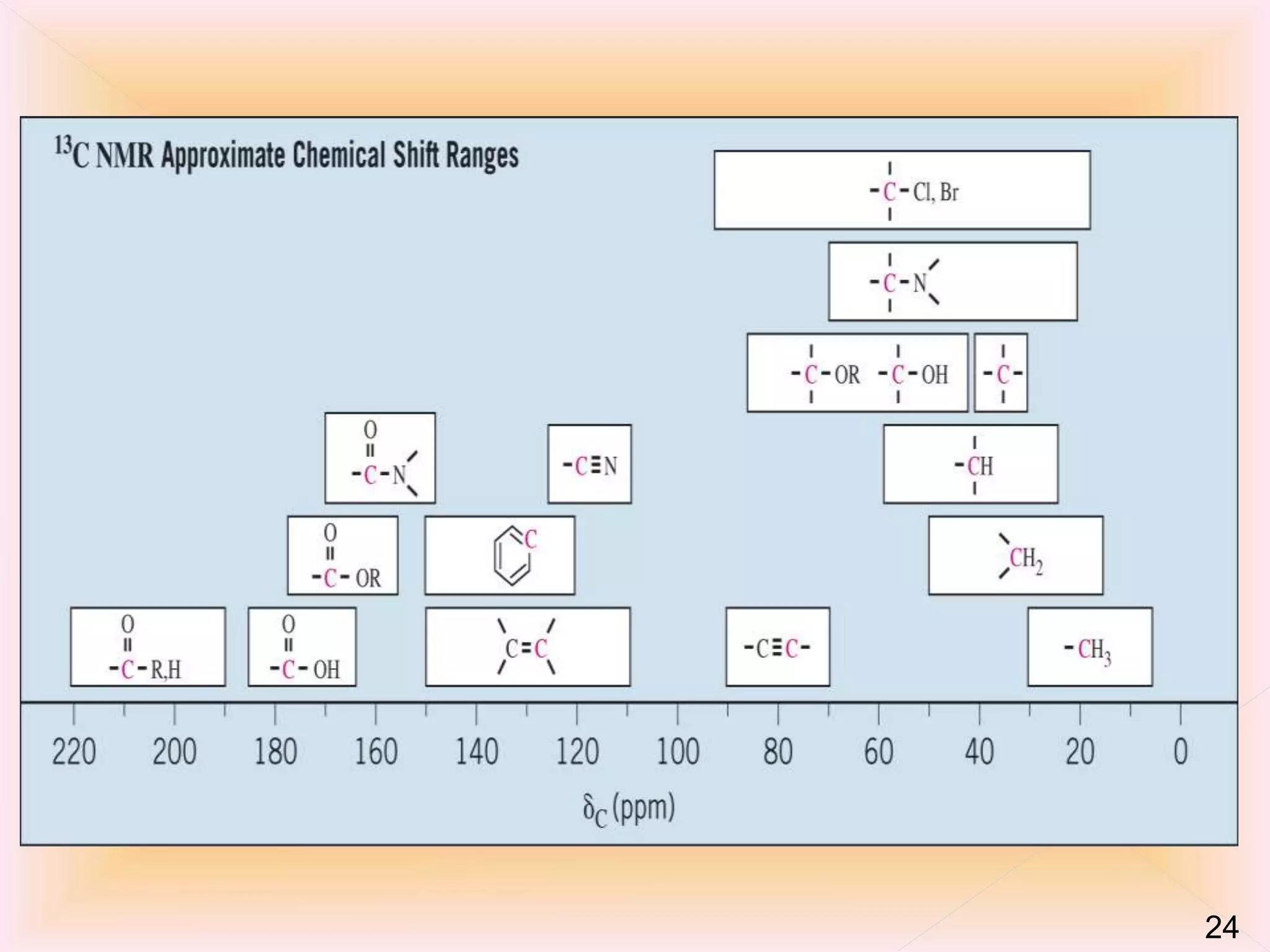
Nuclear magnetic resonance (NMR) spectroscopy can detect certain atomic nuclei that have spin, including the carbon-13 isotope. While carbon-12 does not produce a signal in NMR due to having no spin, carbon-13 accounts for about 1.1% of naturally occurring carbon and can be detected. Carbon-13 has a very weak signal that is difficult to detect due to its low natural abundance and sensitivity being 1/5700 of hydrogen-1. However, the development of Fourier-transform NMR and signal averaging techniques have allowed the detection and analysis of carbon-13 NMR spectra.