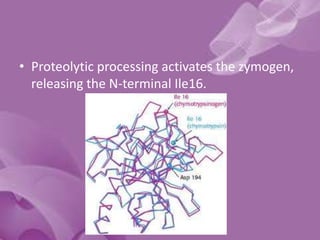
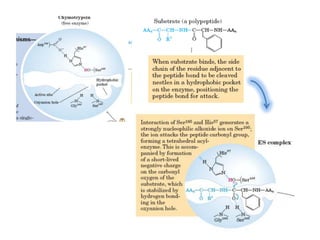
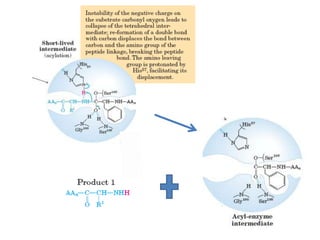
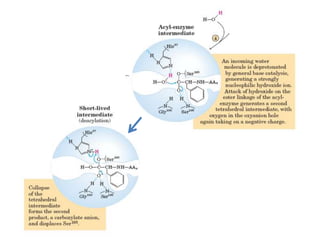
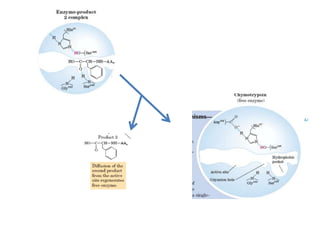
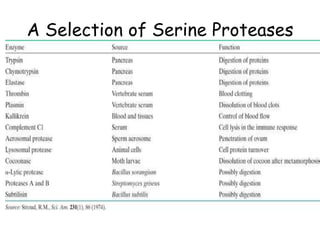
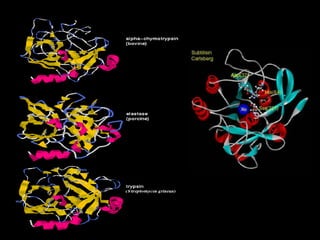
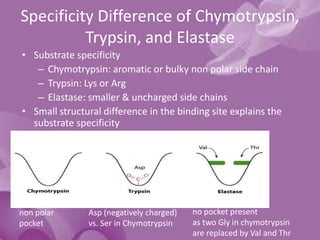
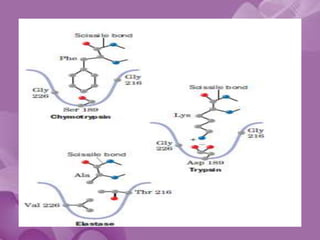
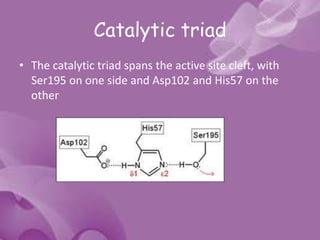
Serine proteases are a large family of enzymes characterized by a serine residue at their active site that cleaves peptide bonds. They include pancreatic proteases like trypsin and chymotrypsin, which differ in specificity and regulation based on structural variations in their active sites. The enzymes operate through a catalytic mechanism involving a catalytic triad and oxyanion hole, with certain proteases synthesized as inactive zymogens that are activated through proteolysis.










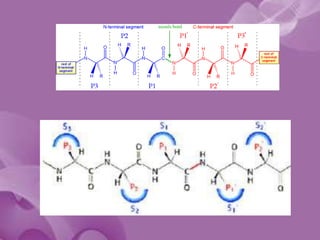
![• Schechter and Berger [1] labeled amino acid
residues from N to C term of the polypeptide
substrate (Pi, ..., P3, P2, P1, P1', P2', P3', ..., Pj)
and their respective binding sub-sites
Si,..., S3, S2, S1, S1', S2', S3',..., Sj) . The
cleavage is catalyzed between P1 and P1'.](https://image.slidesharecdn.com/serineprotease-140407002013-phpapp02/85/Serine-protease-18-320.jpg)