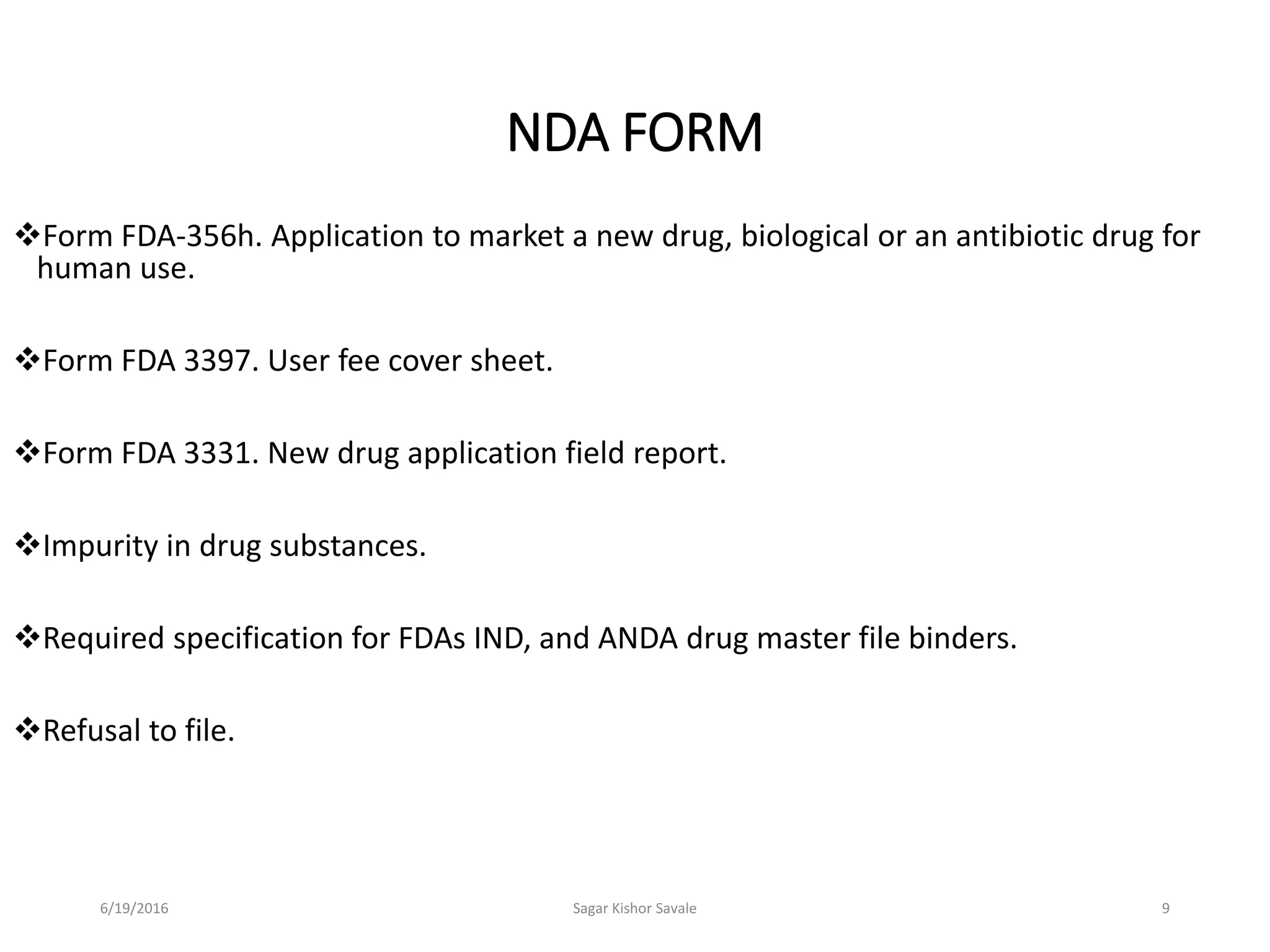
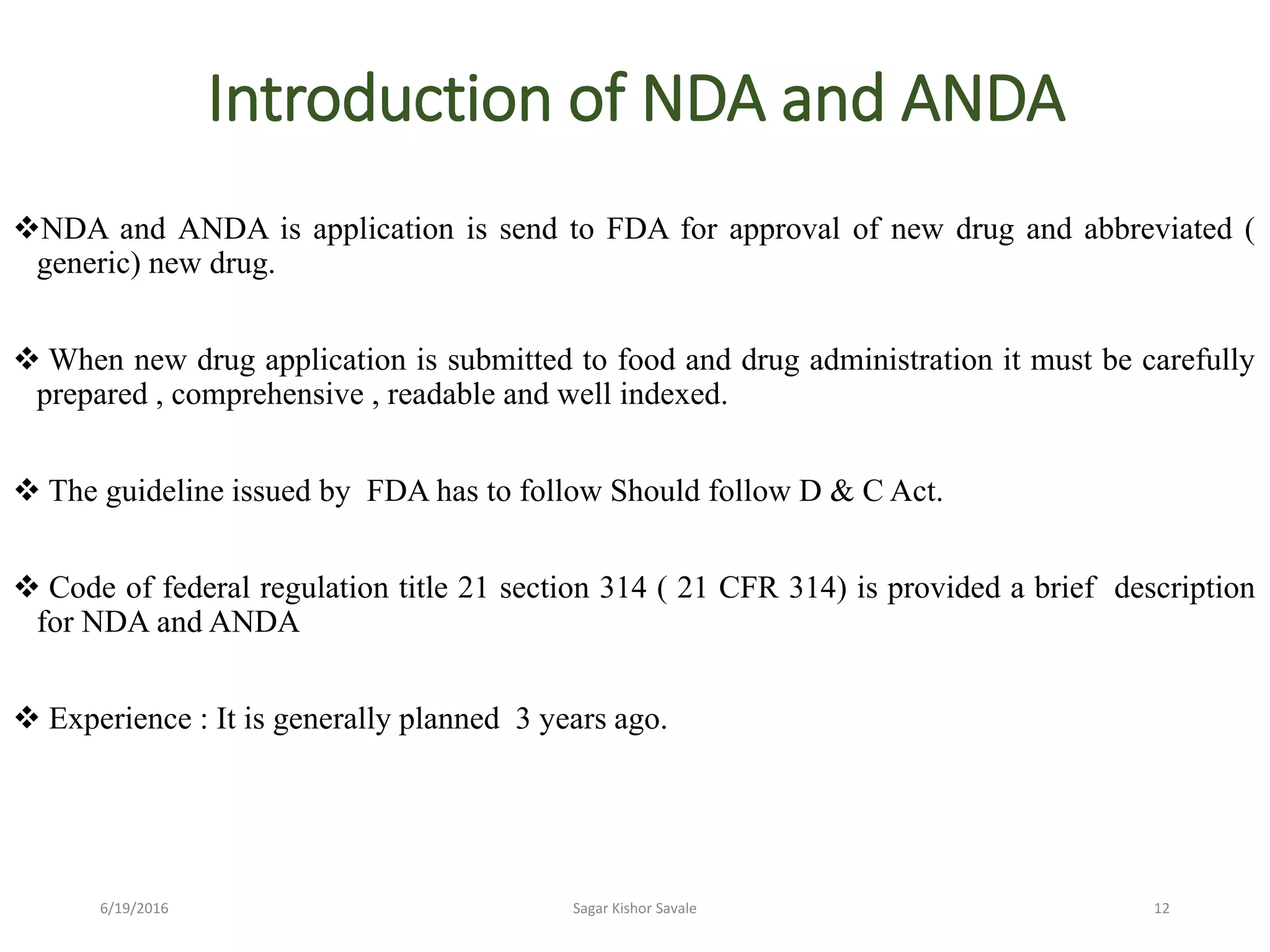
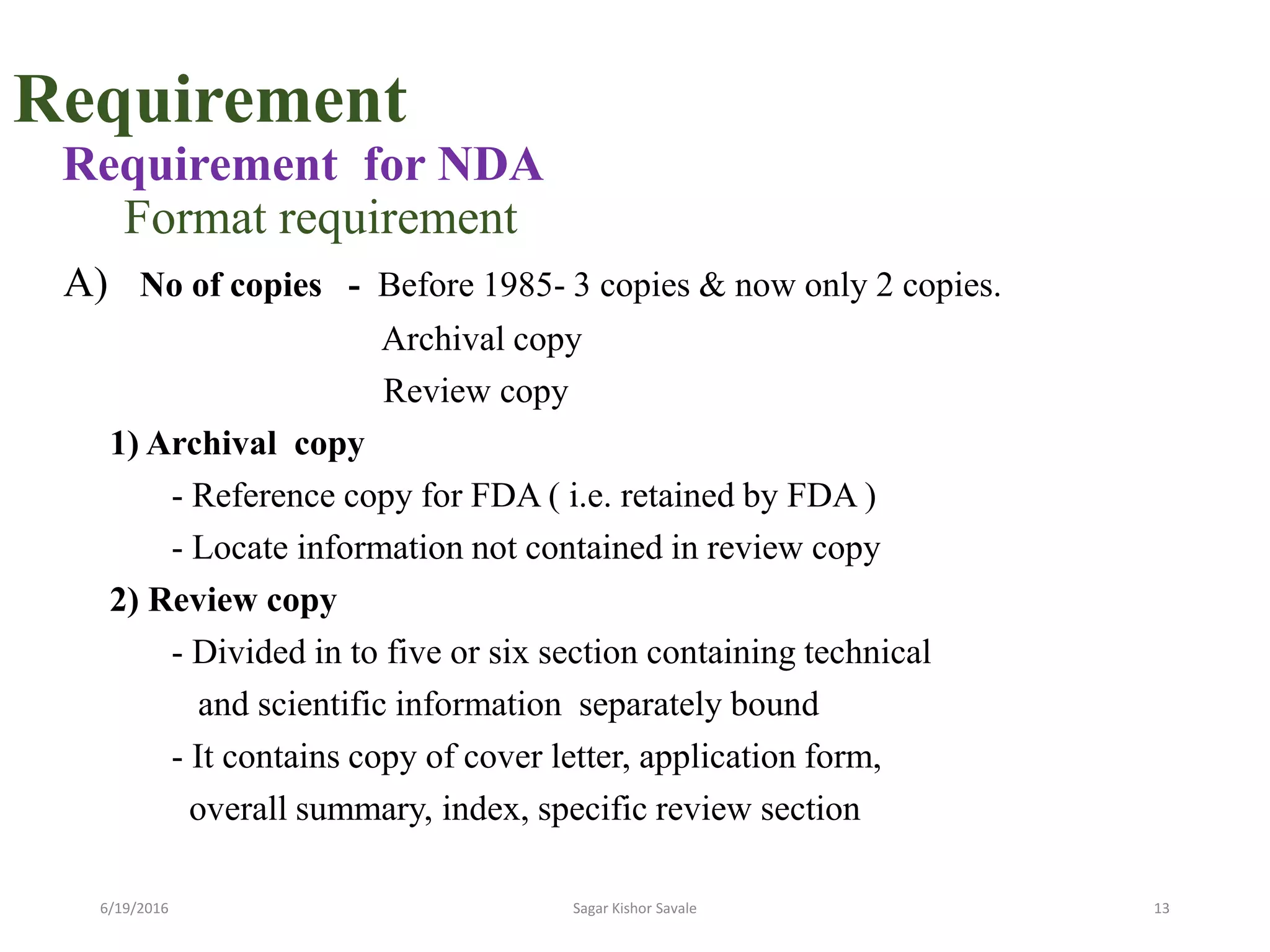
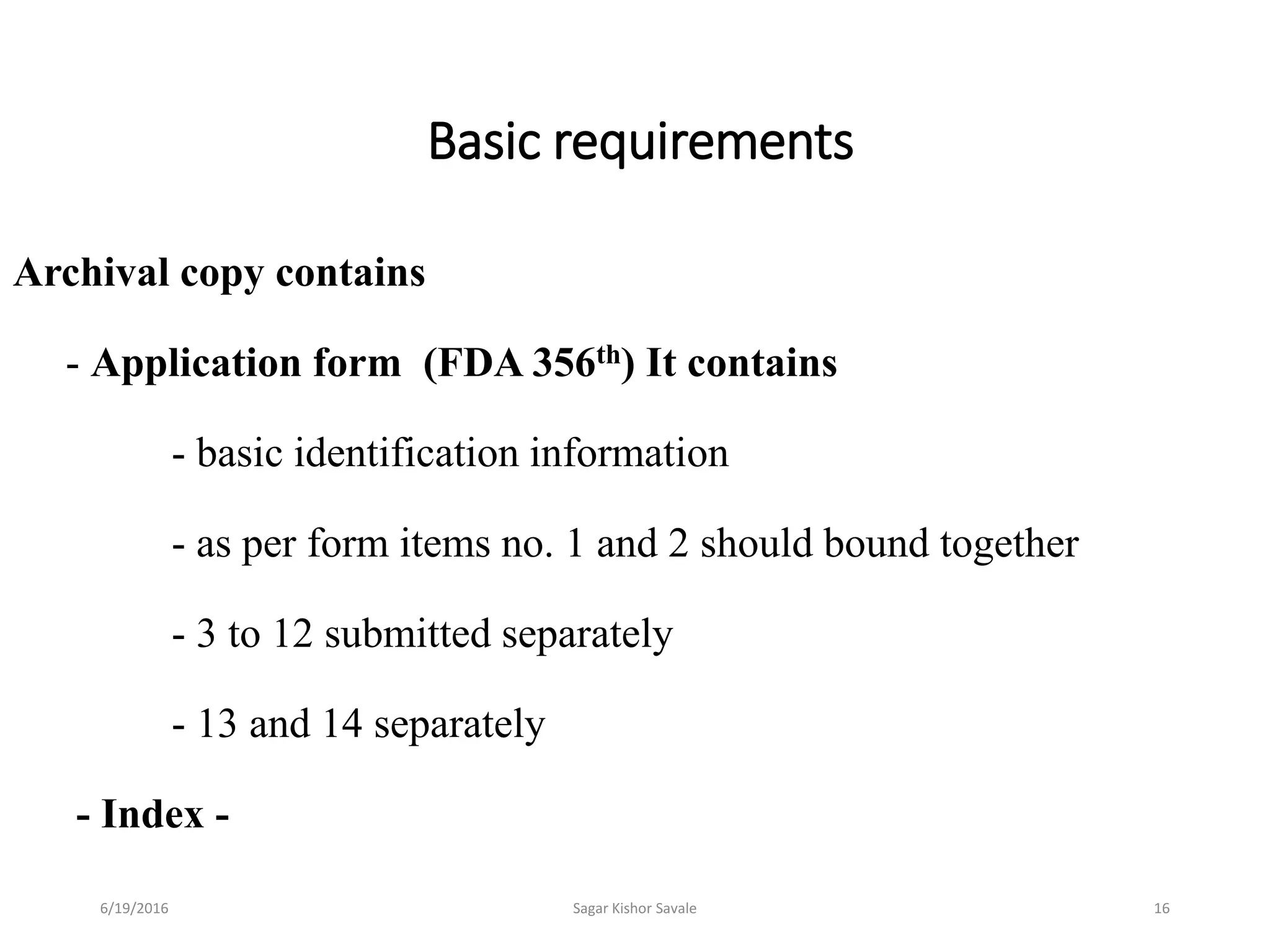
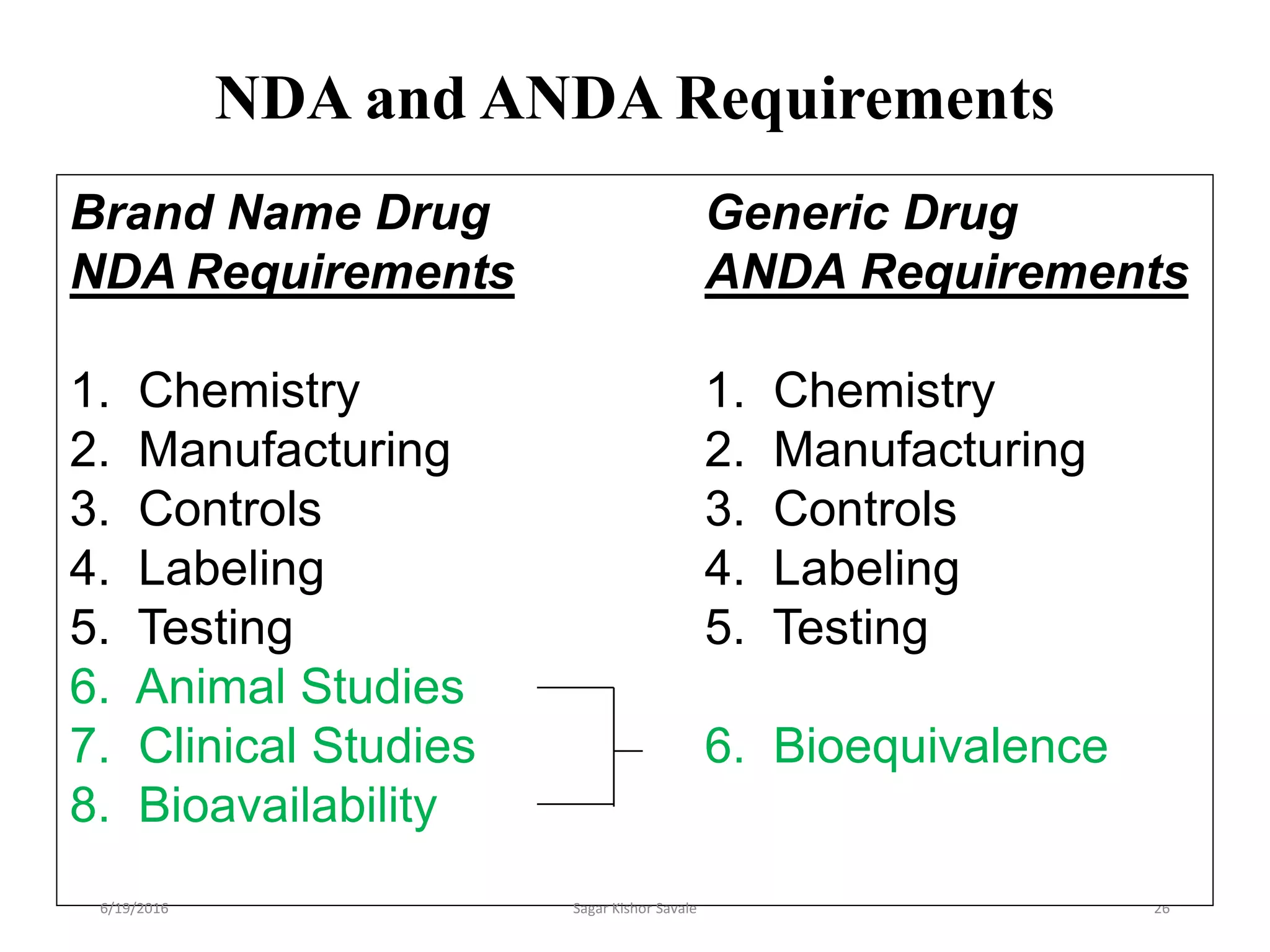
The document outlines the New Drug Application (NDA) process in the United States, detailing its significance, requirements, and the review procedure by the FDA. It explains the necessary documentation, classifications, and the role of NDA in ensuring the safety and efficacy of new pharmaceuticals before market approval. Additionally, it highlights the differences between NDA and Abbreviated New Drug Application (ANDA) for generic drugs.
![1
New Drug Application[NDA]
Mr. Sagar Kishor Savale
[Department of Pharmaceutics]
avengersagar16@gmail.com
2015-2016
Department of Pharmacy (Pharmaceutics) | Sagar Savale
6/19/2016 Sagar Kishor Savale](https://image.slidesharecdn.com/newdrugapplicationnda-160619063242/75/New-Drug-Application-NDA-1-2048.jpg)