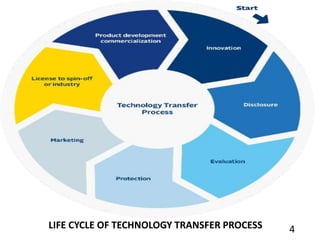
This document provides information about technology transfer in the pharmaceutical industry. It defines technology transfer as the logical procedure that controls the transfer of any process along with documentation and expertise between development and manufacturing sites. The WHO guidelines for technology transfer provide a flexible framework to guide the transfer process with a focus on quality. The scope of the WHO guidelines includes guidance for transferring manufacturing processes of APIs, packaging, and all dosage forms. Reasons for technology transfer include a lack of manufacturing capacity, resources, or marketing capabilities.