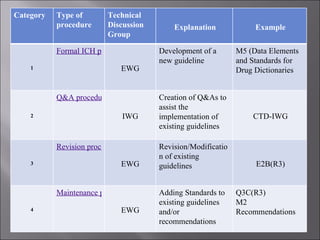
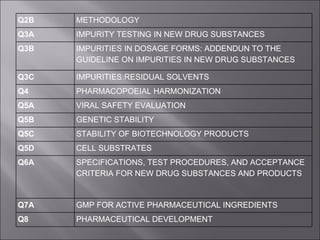
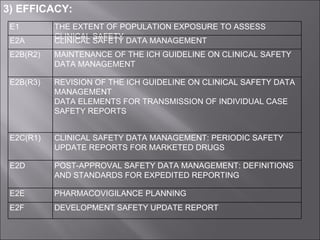
The International Conference on Harmonization (ICH) aims to harmonize technical requirements for pharmaceutical registration across Europe, Japan, and the United States to reduce duplication of testing and delays in availability of new medicines. ICH addresses quality, safety, efficacy, and multidisciplinary topics through guidelines developed by experts from regulatory authorities and the pharmaceutical industry in the three regions. The objectives of ICH harmonization are more efficient use of resources and protection of public health while eliminating unnecessary delays in the global development and availability of new medicines.