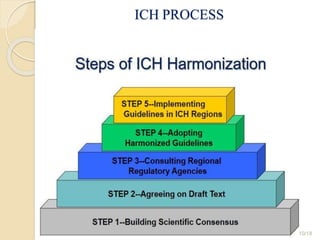
This document provides an overview of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). It discusses the need for harmonization, the origin and evolution of ICH, its objectives and members. It also describes the ICH guidelines, which are categorized into quality, safety, efficacy, and multidisciplinary guidelines. The quality guidelines cover topics like stability testing and impurities, while the safety guidelines address preclinical testing. The efficacy guidelines focus on clinical studies. Overall, the document aims to harmonize technical requirements for pharmaceutical registration internationally.