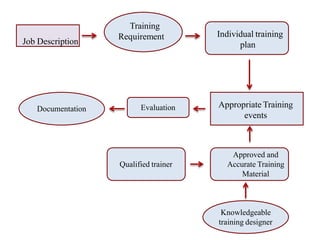
The document outlines the importance of a robust quality assurance system in pharmaceutical manufacturing, emphasizing that qualified personnel are crucial for achieving product quality, safety, and efficacy. It details the roles and responsibilities of key personnel, including the Head of Production (HOP), Head of Quality Control (HOQC), and Authorized Person, along with the necessary qualifications and training for effective operations. Furthermore, it highlights the significance of hygiene practices, training protocols, and maintaining comprehensive personnel records to ensure compliance with Good Manufacturing Practices (GMP).