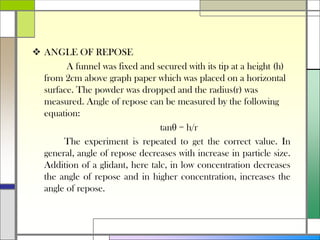
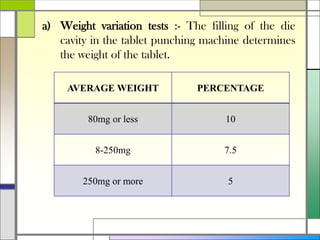
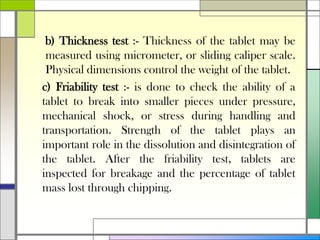
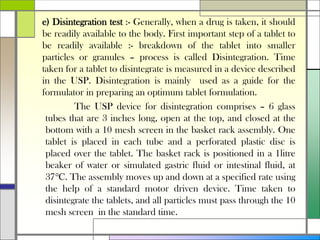
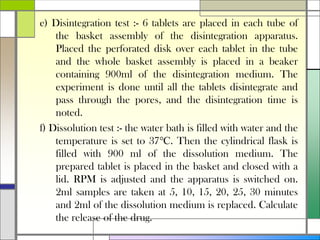
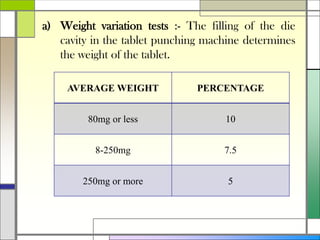
The document describes preparing and evaluating paracetamol granules using the wet granulation method. Key steps in the wet granulation process are outlined, including weighing and sifting powders, blending, wet granulation, drying, dry screening, and lubrication. Granules containing 500mg of paracetamol per tablet are prepared and submitted. The granules are then evaluated for bulk density, tapped density, Hausner's ratio, Carr's compressibility index, and angle of repose to determine flow properties before compression into tablets.